In pancreatic cancer, which has one of the lowest five-year survival rates among cancers, some good news may come from early detection of those arising from cysts.
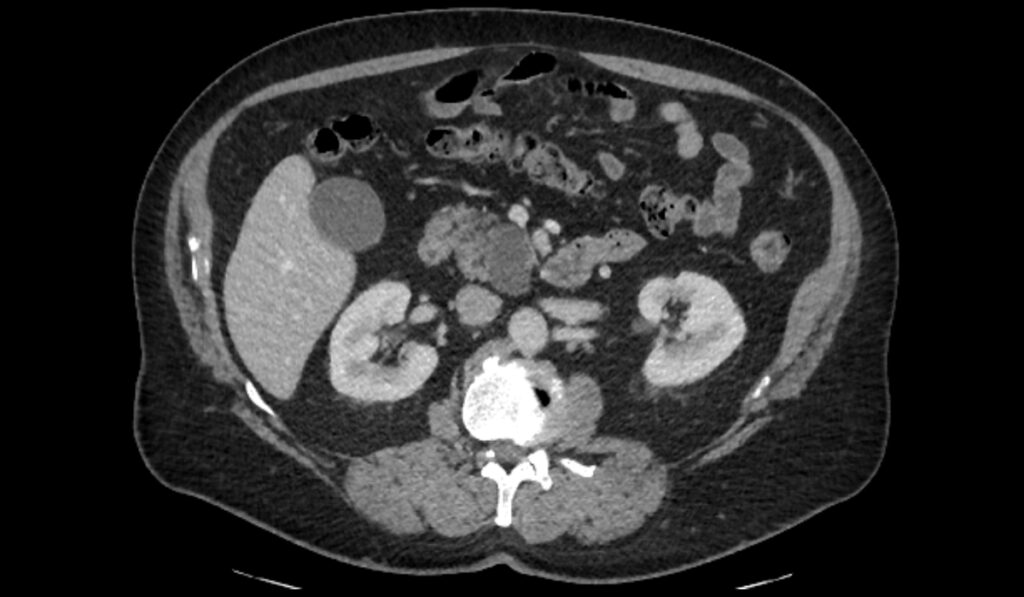
Representing about 20 percent of all pancreatic resections, pancreatic cystic tumors can be more readily detected than the more common subgroup of solid pancreatic tumors. Early detection, however, is marginally helpful in and of itself because benign cysts are common.
Marcus Tan, M.D., an oncologist and pancreatic surgeon at Vanderbilt University Medical Center, is working to identify predictive biomarkers of progression to cancer in these cysts.
“So many more patients and people in the general population are getting scanned these days that we are identifying more of these cysts,” Tan said. “Probably one in 20 people over the age of 60 have multiple cysts in their pancreas.”
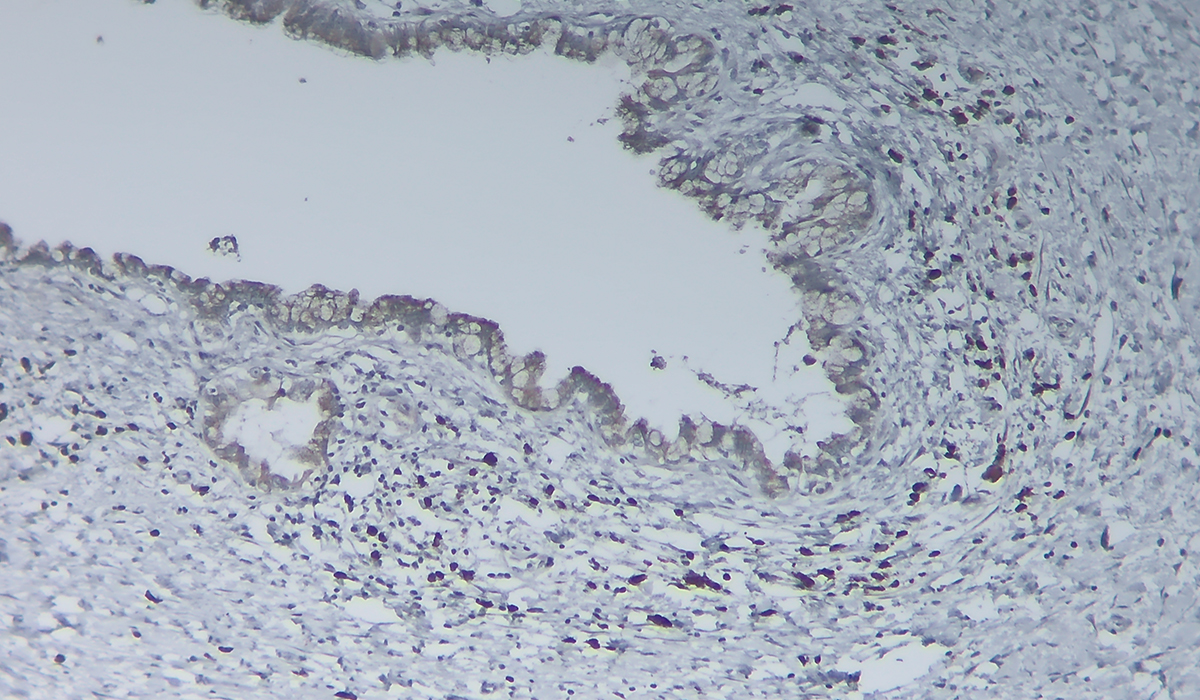
Tan is senior author on a study recently published in The Journal of Pathology which probed how neural cell density in the walls of pancreatic cysts changes over time and whether these changes may be associated with carcinogenesis.
Averting Inappropriate Treatment
The specific cysts Tan studied are premalignant intraductal papillary mucinous neoplasms (IPMNs), which he says are the origin of 5 to 25 percent of pancreatic adenocarcinoma cases. Studying IPMNs may provide clues to how precursors of solid tumors, termed pancreatic intra-epithelial neoplasia (PanIN), turn into cancer as well.
“We wanted to see how the density of nerves in the tumor stroma changed as these tumors grew from early precancerous to late precancerous, and then to actual cancer.”
IPMNs are highly heterogeneous and recognized to have three different subtypes – pancreato-biliary, gastric, and intestinal – each with different rates of malignant transformation. Once invasive disease has arisen from IPMN, it typically behaves just as aggressively as a solid tumor.
“Unlike the case with solid tumors, we can image these cysts early on and follow them. Because of this, there is currently an overtreatment bias in patients with IPMN,” Tan said. “Less than half of patients who have resection of their IPMN over concerns of cancer actually have cancer or even high-grade dysplasia on their final surgical pathology. Given how serious these resections are, we need better guidance on how to identify and monitor suspicious cysts.”
Neural Density as Study Target
The genesis for the study is the growing realization that neural invasion is very important in the carcinogenesis of solid pancreatic cancer, but it had not been studied in IPMN.
“We wanted to see how the density of nerves in the tumor stroma changed as these tumors grew from early precancerous to late precancerous, and then to actual cancer,” Tan said.
These changes were observed developing in the walls of the cysts, where the neural network support resides.
The researchers used multiplexed immunohistochemistry methods and algorithms developed by imaging specialist Joseph T. Roland, Ph.D., a research associate professor in the Department of Surgery at Vanderbilt, to retrospectively analyze and characterize neural development, including sympathetic, parasympathetic and sensory, in 53 cases of IPMN-associated carcinoma across the neoplastic spectrum.
“If we can identify how the tumor and neural precursor cells communicate with the nerves in the stroma, then maybe we can find therapies that interrupt that communication and prevent cysts from progressing to cancer.”
Peculiarities Found in Cystic Neural Density
Three major findings emerged from the study. The first was that there is a significant increase – approximately a doubling – in the neural density as the cyst goes from early precancerous (low-grade dysplasia) to late precancerous (high-grade dysplasia). Similarly, the density of supporting Schwann cells was higher in high-grade versus low-grade IPMN. However, the density of nerves and Schwann cells did not increase further from high-grade dysplasia to frank invasive disease.
The second was that the nerves within the tumor walls were dissimilar to those in solid tumors or in the rest of the pancreas, which have predominantly mature, usually sympathetic nerves. Instead, Tan and colleagues found that the nerves within the IPMN tumors were very immature and lacked the sympathetic markers typical of stromal nerves in solid pancreatic tumors.
This demonstrated that immature nerves, regardless of native type, are a defining feature of pre-invasive disease in IPMNs.
The third finding was the identification of a separate population of neural progenitor cells in these tumors, something Tan says has never been described in any type of pancreatic tumor before.
Toward Identification of Pre-cancers
Tan says these findings raise several prospects for future functional studies in IPMN organoids and mouse models. Such studies will aim to understand the mechanisms underlying neural recruitment into the IPMN stroma and how neural precursors, immature nerves, tumor cells and other components of the tumor stroma interact.
The findings also point toward therapeutic studies to investigate whether a blockade of intratumor neural development will arrest malignant progression.
“From a diagnostic point of view, we’re looking at how the various neural chemicals or neurotropic factors may increase in the cyst fluid as you go from early precancerous to high precancerous to cancer,” Tan said. “If we can identify how the tumor and neural precursor cells communicate with the nerves in the stroma, then maybe we can find therapies that interrupt that communication and prevent cysts from progressing to cancer.”