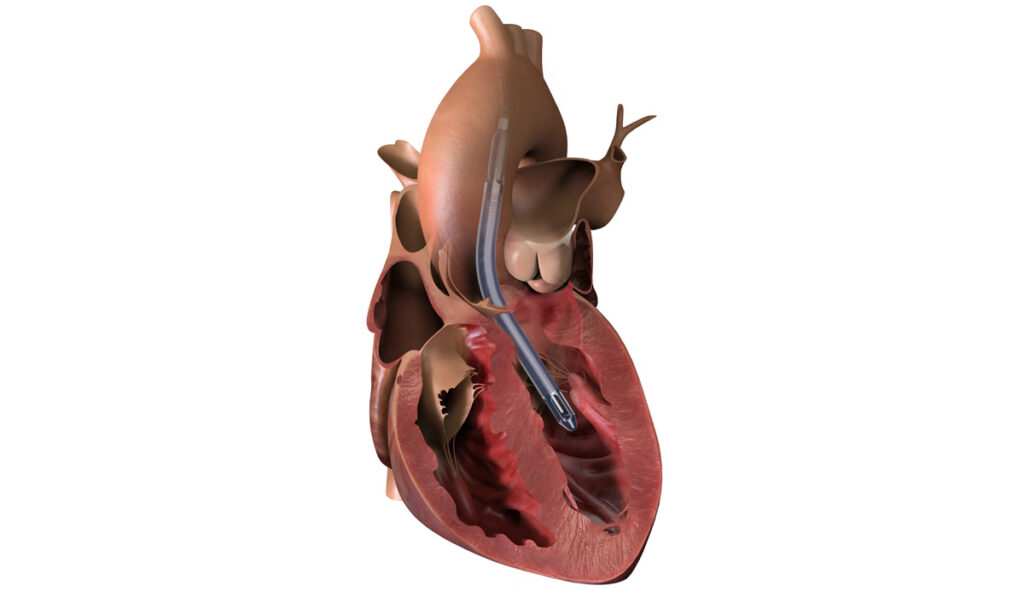
The Impella® ventricular assist device (VAD), used as a rescue therapy since 2008 for adults with cardiogenic shock following heart attack, is now providing similar benefits to a subset of pediatric patients.
At Monroe Carell Jr. Children’s Hospital at Vanderbilt, David Bearl, M.D., medical director of the VAD program, and Justin Godown, M.D., medical director of pediatric cardiomyopathy, after carefully reviewing the literature are poised to offer the implant to children and adolescents who meet size and clinical criteria.
Additional evidentiary support for pediatric use of the device, manufactured by Abiomed, springs from collaborations within the multi-institutional Advanced Cardiac Therapies Improving Outcomes Network (ACTION).
“This device is able to provide ventricular support percutaneously without doing a major surgery, while offering patients one or two weeks, or longer, for their left ventricle to rest or recover,” Bearl said.
Trends in Pediatric VAD Use
Pediatric candidates for this left ventricular assist device (LVAD) are those who have acute decompensated heart failure largely due to malignant arrhythmias, transplant rejection or myocarditis.
Randomized controlled trials in pediatrics are extremely scarce, but retrospective studies demonstrate that a VAD can effectively mitigate the severity of illness in children. Patients assisted by VAD, compared to others who are not, are four times more likely to survive to transplant.
Further, ACTION centers report that the number of patients discharged home with an LVAD has increased over time. While readmissions for diverse indications are common, the risk of mortality during rehospitalization is low. At Vanderbilt, the first child was sent home with an LVAD in 2019. Overall, the frequency of discharge across the 25 ACTION centers increased 62 percent between 2009 and 2018.
Impella for Short-term Support
Bearl explains that the Impella is not indicated for home use. Rather, it is typically used in patients expected to recovery rapidly but still need support for a few days. In other cases, it may be part of a patient’s progression to durable VAD or may become a bridge to transplant.
The device is inserted by catheter from the axillary artery into the aorta and down into the left ventricle. From there, a micromotor at the end pulls blood up from the ventricle distal to the aortic valve, where it is dispersed into the aorta. This unloads ventricular pressure and provides short-term cardiac output for heart perfusion and systemic support.
The width of the patient’s axillary artery must be 7 millimeters or greater, limiting candidates to adolescents and larger children.
“The Impella provides sufficient cardiac support to compensate for a failed left ventricle in a resting patient while we wait for recovery,” Bearl said.
“Other ventricular assist devices that we have require a full sternotomy, with the patient going on bypass, where this does not require any of that,” Bearl said. “This just potentially needs a small graft to the axillary artery, and this entry point allows for greater mobility and lowers the risk of limb ischemia.”
Alternative for ECMO
The Impella may also work as a substitute for extracorporeal membrane oxygenation (ECMO) or as a companion therapy to improve hemodynamics.
Brian C. Bridges, M.D., medical director of ECMO at Children’s Hospital, said the device provides especially good options for myocarditis treatment.“We are potentially interested in using it to support VA-ECMO instead of doing balloon atrial septostomy or left atrial vent,” Bridges said.
ECMO carries a significant stroke risk. However, using Impella to support cardiac output excludes the need for an oxygenator, reducing those overall risks.
“The Impella provides sufficient cardiac support to compensate for a failed left ventricle in a resting patient while we wait for recovery.”
More Comprehensive Training
Bearl, Nancy Jaworski, D.N.P., VAD coordinator at Children’s Hospital, and the Abiomed staff have led 180 Vanderbilt staff members through Impella training, and the devices are on the shelf, ready for use.
“Because it is placed percutaneously, all of our cath lab staff needed to be trained,” Bearl said. “Our OR staff and our intensivists all needed to be prepared to manage this device. Cardiac intensivists have to be in the ICU, checking on the patients hourly, prepared to make adjustments.”
Further, he said the nursing staff required separate training on dressing care and charting, and the sonographers had to be trained on specific criteria for the echocardiograms.
“It’s important that any center embarking on this understands that while it is less invasive than the other VADs, it requires a broader range of staff to be trained,” he said. “It will have all been worthwhile once we see that first patient spared a major VAD surgery.”