Through his research and practice, Matthew Bacchetta, M.D., is tilting at a dragon larger than almost any other medical problem in the United States: chronic lung disease.
More than 3 million people die annually from the disease, a number that has been increasing even as the top two killers, heart disease and stroke, are trending downward.
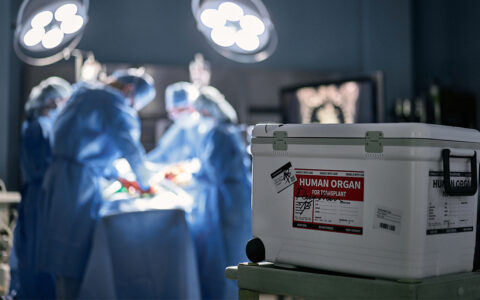
In advanced cases, a patient’s best alternative often is a lung transplant, but the lack of viable organs is a major hurdle. Only about 2,500 lung transplants take place each year in the United States due to the shortage of donors and the lack of high-quality organs to replace the failing ones.
“There’s simply a severe shortage of donor organs in general,” said Bacchetta, the H. William Scott Jr. Chair in surgery at Vanderbilt University Medical Center and chief surgical officer of the Vanderbilt Lung Institute. “Because of that, it has become a complex process which has made lung transplantation very selective.”
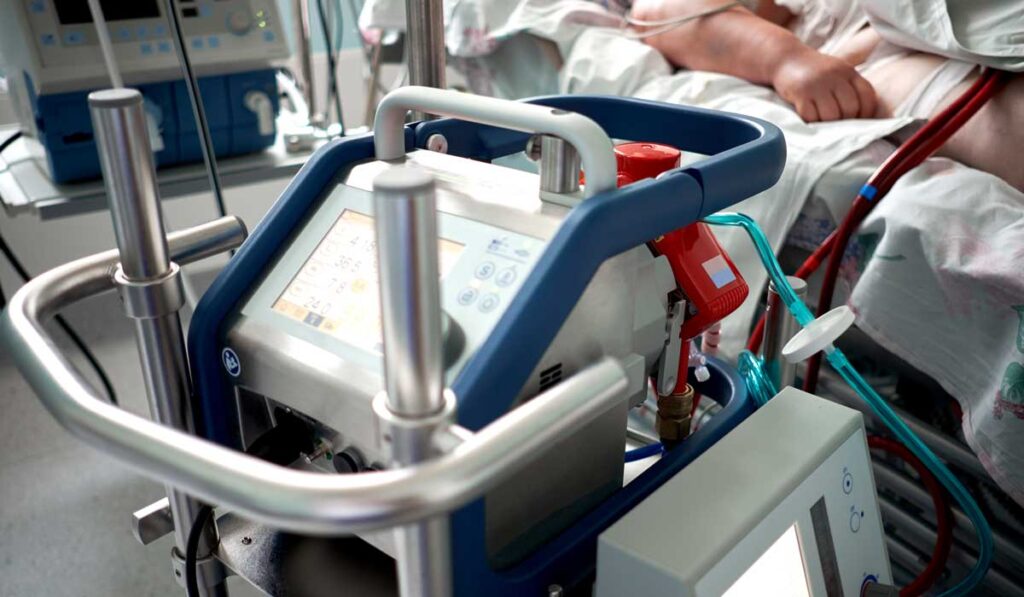
Management of the disease without a transplant may require extracorporeal membrane oxygenation (ECMO) while a patient’s own lungs heal. To date, this treatment is only available in intensive care units, but that may change following an initiative to create a simpler, more portable ECMO.
Remodeling the Platform
Bacchetta, along with colleagues at Carnegie Mellon University and Cornell University, has been awarded $1.47 million by the U.S. Department of Defense to conduct ECMO research in response to a high level of lung disease among service members.
They plan to modify the bulky, complex ECMO device to enable patients to avoid weeks in the hospital’s intensive care unit.
“The first goal is to get them out of the ICU,” he said. “We can’t move them from the ICU now because the technology is too complex and the equipment is too heavy. It takes a small army of people to mobilize a patient attached to an ECMO machine.”
A smaller, lighter-weight device not only would allow patients to be treated on a regular hospital unit but, potentially, in their own homes.
“If we can, we will further refine it to let people go home with the treatment, as we do with a ventricular assist device,” he said. “This allows them to recover outside the hospital and get back to a more regular life.”
Help for Chronic Disease
Since its development in the 1960s, ECMO has become a lifesaver for many patients, not only those with chronic lung disease.
“There are several indications for ECMO, including pulmonary disease or primarily cardiac disease,” Bacchetta said. “If the pulmonary disease is acute, like a bad pneumonia, ECMO can be a bridge to recovery.”
Approximately 16 million Americans have been diagnosed with chronic obstructive pulmonary disease, or COPD, and millions more are believed to have the disease yet remain undiagnosed.
“We only use about 20 percent of the donor organs we are offered, because some are just not good enough.”
In the course of COPD, the physical discomfort typically starts slowly.
“At first, people will accommodate by being on a little oxygen, but that will worsen over time,” Bacchetta said. “Eventually, they lose the ability to conduct typical activities of daily living and can become very restricted, essentially homebound, and increasingly susceptible to hospital admissions.”
Demand Exceeds Supply
In many cases, lung transplant becomes the only option. But with so many patients and so few available lungs, every patient is highly scrutinized before being added to the transplant list. In most cases, recipients must be younger than 75, in otherwise good health, fit enough to participate in rehabilitation, and abstinent from tobacco and alcohol use, among other qualifying characteristics.
With the advent of COVID-19, which causes thrombosis, compromised blood vessels and fibrin structures, inflammation, occlusion of alveolar spaces, and other types of lung damage in many patients, scarcity has worsened.
“The lung shortage was already severe; COVID obviously made things worse,” Bacchetta said.
In 2019, Bacchetta introduced an ex vivo lung perfusion (EVLP) device he helped develop to increase the supply of donor lungs by rehabilitating organs previously considered too damaged for transplant.
“We only use about 20 percent of the donor organs we are offered, because some are just not good enough,” Bacchetta said. “So, we are trying to make the other 80 percent better to offer lungs to more people and have fewer die on the wait list.”
Conditions negatively affecting donated lungs include previous damage, aspiration into the lungs, pulmonary contusions, and deterioration after removal. Vanderbilt is one of a few medical centers in the United States operating EVLP, which has made possible at least an additional 20 successful transplants at the medical center, and is part of a clinical trial studying a more expanded use of the technique.
Vanderbilt’s team will focus on engineering and testing the modes of vascular access and the ergonomics of the device, as well as develop more efficient anticoagulants and refine the gas exchange portion of the device, Bacchetta said.
Challenges will include development of new coatings to allow blood to travel through the device more efficiently and avoid clots. Researchers will also work to develop the remote monitoring system that will enable the patient to leave the hospital. The project has funding of more than $8 million over four years and the results are eagerly anticipated.
“If we had this device in our hands, we’d be using it today,” Bacchetta said.