Long gone is the notion that non-coding RNAs floating throughout the body lack functionality. Instead, growing evidence shows non-coding RNAs not only interact to regulate gene expression, but also contribute to disease pathogenesis.
Michelle Ormseth, M.D., an assistant professor of rheumatology and immunology at Vanderbilt University Medical Center, has been studying small RNA fragments for years. Ormseth and colleagues recently published a summary in Nature Reviews Rheumatology describing the interplay between non-coding RNAs in the synovial joint, a space typically inflamed in rheumatoid arthritis.
“The picture of how the rheumatoid arthritis synovium becomes so inflamed and hyperproliferative is a much more complicated process than we all originally thought,” Ormseth said. “There are so many factors that are contributing to that process.”
Many Players Yield Net Effect
The summary article describes many roles for non-coding RNAs – a broad umbrella term that includes small RNAs like microRNAs as well as long non-coding RNAs and circular RNAs – in joint health and disease. Non-coding RNAs are found inside and outside cells. They can also transfer between cells via extracellular vesicles. Non-coding RNAs like long non-coding RNAs and circular RNAs have functions distinct from that of the more well-known microRNAs, Ormseth explained.
“Typically, we think of microRNAs as attaching to messenger RNA and downregulating a gene of interest. But then there are these other non-coding RNAs that act by ‘sponging.’ They bind to microRNAs to sequester them from having any effect on the target gene.”
Through sponging, some non-coding RNAs compete with microRNAs in the body to control gene expression. The net result of this process can dictate cell differentiation and, eventually, affect arthritis symptoms.
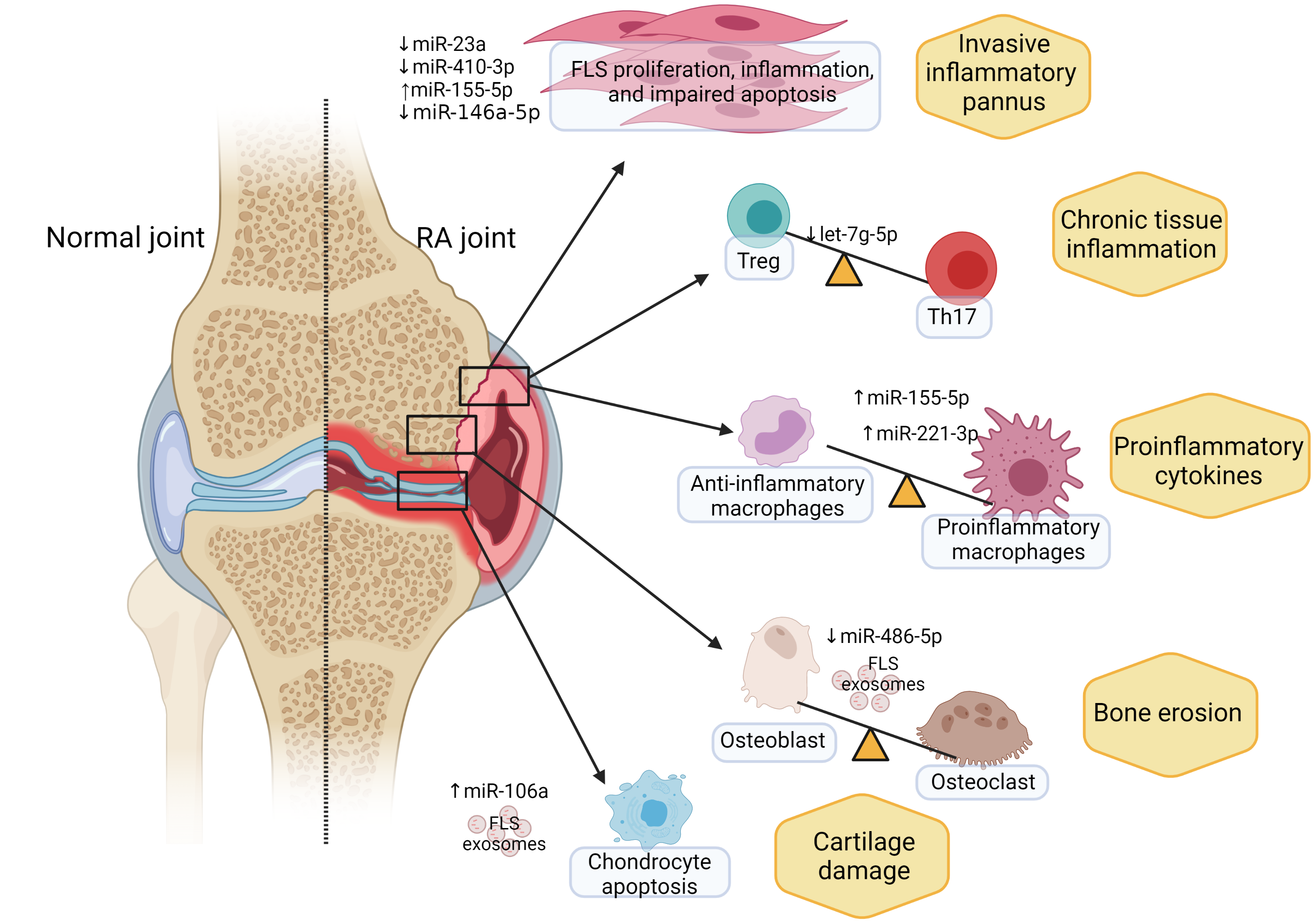
A Dynamic, Inflammatory Process
Ormseth’s team has used a network biology approach to better understand non-coding RNAs in the synovial joint in people with rheumatoid arthritis.
“We collected everything related to non-coding RNAs in the literature, including any known targets and implications,” Ormseth said. “We find a lot of redundant targeting by non-coding RNAs on, for example, the same microRNA or on the same pathway.”
The overall effect of non-coding RNAs in rheumatoid arthritis is an increase in synovial fibroblasts that are highly resistant to apoptosis – a hallmark of the disease.
“The synovium is normally a thin, cellophane-like membrane that goes around the joint to create a lubricant,” Ormseth explained. “In rheumatoid arthritis, that membrane becomes thick and proliferative. Instead of being a thin, single-cell layer, synovial fibroblasts form many layers that then lead to immune cell infiltration and joint swelling.”
Through laboratory models, the researchers have found aberrant non-coding RNAs inside the synovial fibroblasts themselves, inside immune cells, and in transit between the two. “We don’t always know where these non-coding RNAs come from,” Ormseth said.
Beyond Human RNA
Some of the non-coding RNA may not even be human, she said. Other work by Ormseth has shown gut bacteria secrete exosomes containing different kinds of non-coding RNA. These exosomes are elevated in people with rheumatoid arthritis.
“We’re beginning to look at how microbial extracellular vesicles in the gut may be crossing into our bloodstream and how this may be affecting our immune function,” Ormseth said.
One challenge to rapid results is the lack of data on microbial non-coding RNAs required to facilitate a network biology approach, as is possible when studying human RNAs. In essence, Ormseth is starting from scratch when cataloguing microbial non-coding RNAs.
Still, with each non-coding RNA identified and catalogued, whether human or microbial, Ormseth moves a little closer to teasing apart immune responses that underlie diseases like rheumatoid arthritis. And, since the activity of non-coding RNAs can be modulated via small molecules or exosomes, the work may reveal attractive therapeutic targets.
“Non-coding does not mean non-essential,” she said. “We’re beginning to understand that non-coding RNAs are critical regulators of inflammation and inflammatory disease.”