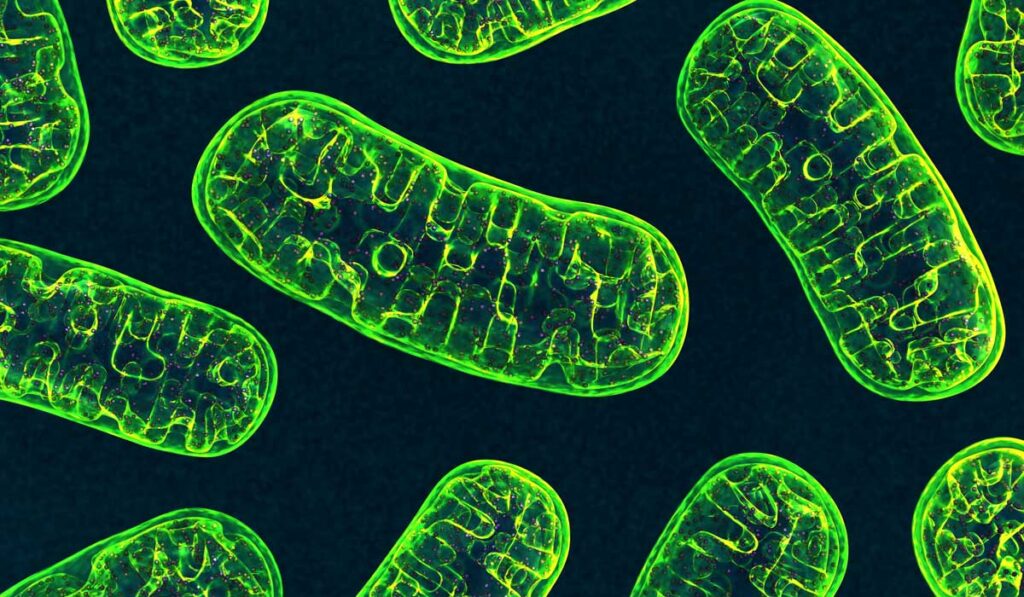
Using structured illumination microscopy (SIM), researchers at Vanderbilt University Medical Center have uncovered new insights into the 3D morphology of mitochondria, starting with the kidney. The findings, published in Kidney360, suggest that mitochondrial interconnectivity protects against kidney injury.
Craig Brooks, Ph.D., a cell biologist and microscopy expert at Vanderbilt, and his team of researchers imaged mitochondria and other organelles from mouse tissue and human kidney biopsies. Their super-resolution imaging technique allowed images to be 3D-rendered to quantify mitochondrial size, content and morphology.
“SIM imaging wasn’t originally designed to view tissue samples,” Brooks said. “Previously, the only way to view the kidney was through the use of labor-intensive techniques, such as electron microscopy. This is the first time we’ve used SIM to visualize mitochondria in human kidney samples.”
Super-resolution Imaging
Among the super-resolution imaging techniques, SIM is attractive because it can be performed on samples stained using standard immunofluorescence techniques and confers double the resolving power of standard light microscopy. It can be used to view various cellular organelles and structures.
“Promoting mitochondrial fusion maintains mitochondrial morphology and thereby reduces kidney injury.”
The researchers hypothesized that SIM could be used to create 3D models of mitochondria from cells in kidney tissue, allowing for the quantification of mitochondrial network size and morphology. In their analysis, they combined organelle markers with injury or cell-type specific markers and looked for patterns.
3D Mitochondrial Morphology
Super-resolution imaging revealed that mitochondria form large, interconnected networks in kidney tubule cells, indicating that under basal conditions, kidney tubule cell mitochondria represent a single, continuous membrane structure.
Brooks explained that while this finding appears inconsistent with the classical textbook description of mitochondria, it is in line with findings in other organs and cultured mammalian cells.
“Our analysis utilized surface renderings of organelles, allowing for the digitization of each organellar unit and providing values and statistics of various features of the organelle.”
Using these data, Brooks connected a loss of mitochondrial volume with injury, but also quantified the level of fragmentation of the mitochondria network in mouse and human kidney tissue.
Further co-staining with the injury marker KIM-1 revealed that while injured proximal tubule cells have the largest reduction in mitochondrial volume and most mitochondrial fragmentation, KIM-1 negative (and presumably healthier) proximal tubule cells also had a significant reduction in mitochondrial volume. Promoting mitochondrial fusion not only maintained mitochondrial morphology, volume and interconnectivity, it also improved cisplatin-induced kidney injury.
“Our results suggest that promoting mitochondrial fusion maintains mitochondrial morphology and thereby reduces kidney injury,” Brooks said.
Future Applications
While electron microscopy remains the gold standard for analyzing organelle morphology in kidney tissue, Brooks emphasized that it is vital to measure mitochondrial swelling, cristae alterations, autophagy, lysosomal disruptions and other features. Super-resolution imaging offers an alternative to standard, labor-intensive examination techniques.
Brooks acknowledged that super-resolution techniques cannot provide detailed information on cristae structure and other fine ultrastructural details. Thus, he recommended that super-resolution imaging be used in parallel with or to augment electron microscopy studies.
“In the future, SIM imaging and analysis techniques could be used to quantify organellar dynamics of multiple organelles in kidney tissue and other organs, such as the heart, in the research setting and potentially in the clinic.”