First discovered in the 1920s, Raman spectroscopy has only recently evolved from a laboratory tool to one with the potential to guide clinical decisions.
Jeffry S. Nyman, Ph.D., an associate professor of orthopaedic surgery at Vanderbilt University Medical Center, is working to incorporate the technology preoperatively or intraoperatively for surgical decision-making, such as whether fixation versus joint replacement is preferable for older patients with a fracture.
In Nyman’s laboratory, in collaboration with the Vanderbilt Biophotonics Center, researchers are using fiber-optic Spatially Offset Raman Spectroscopy (SORS) to measure changes in the extracellular matrix (ECM) of bone.
“We’re working to be the first to show that SORS is sensitive to matrix changes that directly relate to the loss of strength,” he said.
Deterioration of the ECM reduces the fracture resistance of bone. However, this does not necessarily correlate with bone mineral density, which is measured by traditional dual-energy X-ray absorptiometry (DEXA) scans. The group’s aim is to incorporate SORS in addressing fracture resistance to create a more fully informed and stratified diagnosis of osteoporosis than is currently possible.
“Bone mass, as measured by DEXA scans, is not the whole picture,” Nyman said. “We want to shift the paradigm of bone health from a focus on this characteristic alone to the inclusion of pathogenic contributions from the organic matrix of the bone.”
Vibrations Tell a Complex Tale
Raman spectroscopy is a light scattering technique that reveals multiple physical and chemical properties of any material. By stimulating molecules and then graphically representing the vibrations, this method identifies the types of molecular bonds present and yields mineral-to-matrix ratios, as well as the crystallinity and quantities of carbonate substitution in the bone mineral.
“Taken together, this gives us a projection of matrix quality that DEXA cannot offer. It provides information about the toughness of bone versus just knowing the amount of bone mineral,” Nyman said.
Clean Waves
His group’s first challenge was discerning what portion of the detected Raman spectra represented significant markers of the bone’s mechanical properties.
“It’s not just about identifying molecules by their vibration,” Nyman said. “You want to also find out which characteristics are clinically meaningful in terms of predicting fracture risk.”
Part of that includes separating “clean” waves from bone tissue from other tissues such as skin, fat and even the periosteum which “contaminate the signals coming from the bone,” Nyman said.
“We want to shift the paradigm of bone health from a focus on this characteristic alone to the inclusion of pathogenic contributions from the organic matrix of the bone.”
The SORS probe was specifically developed to counter this noise. It enables the collection of light from specific depths, so the bone waves can be isolated from those emanating from overlying tissues.
Putting the Probe to the Test
To test the sensitivity of their Raman output, Nyman and colleagues put cadaveric femur bones through an autoclaving manipulation that weakened the bone. The researchers then measured ECM-related properties of both the autoclaved bones and unmanipulated controls first at the surface level, and then again after consecutively adding layers of tissue phantoms, up to about 10 mm.
“The Raman spectra showed, first, that it detected the bone alteration and, second, that it could measure the ECM properties at certain distances from the surface with the same results,” Nyman said.
“For the most part, we felt the Raman probe was good for a site in which bone was three millimeters below the surface, sometimes four,” Nyman said.
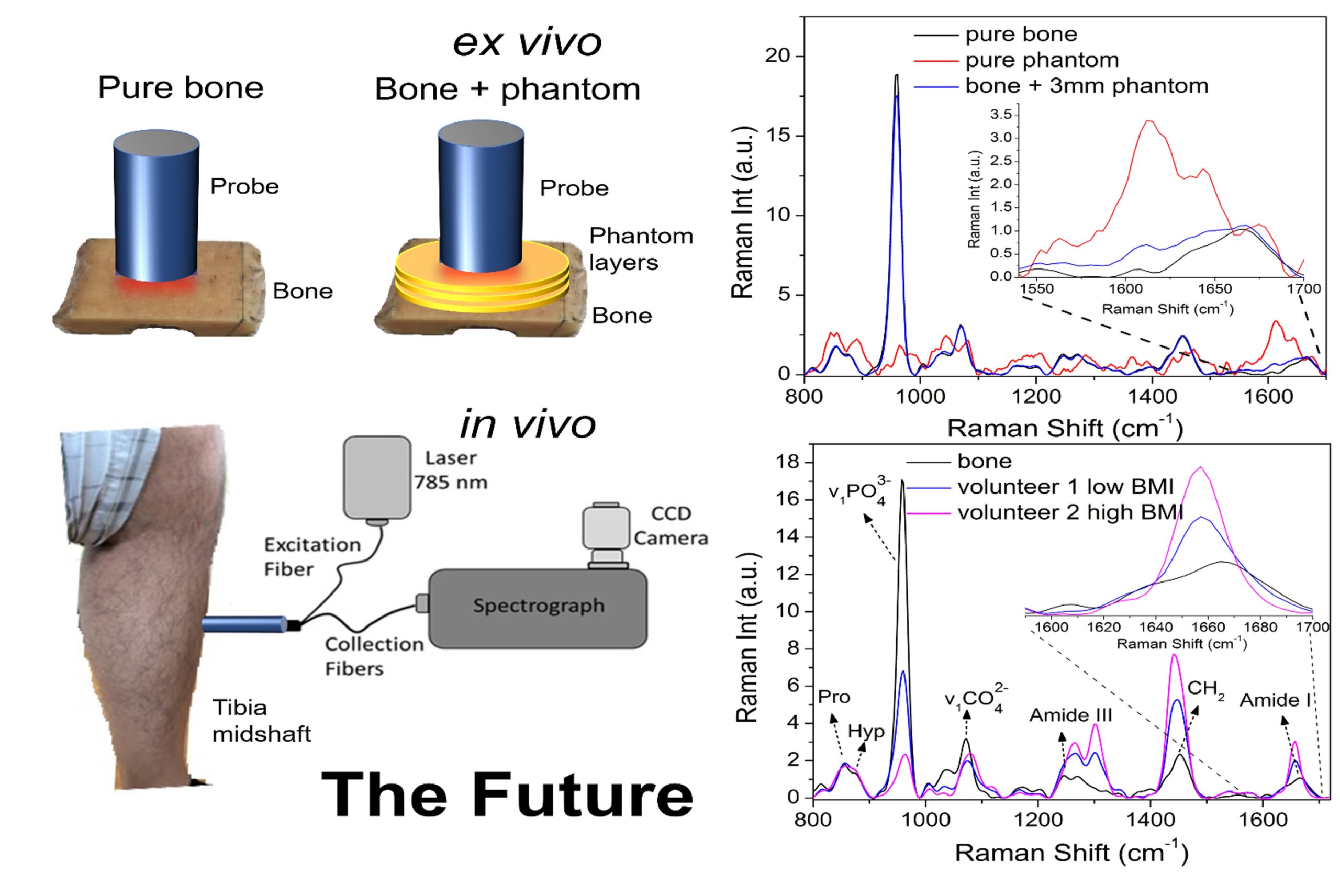
Clinical Experimentation
With these depth limitations in mind, the anteromedial area of the shin and an area of the kneecap where there is a flat surface are the closest contacts that the Raman probe can make with bone in vivo – about 2 mm in a person who doesn’t have a high BMI, Nyman says.
He is zeroing in on shin bone measurements to learn if results there would be representative of a person’s overall bone strength. To do this, he plans to do a cross-sectional study on patients admitted to the hospital with a hip fracture.
“We will look at patients with a bona fide osteoporotic fracture, compare with age-matched individuals with no history of fracture and see if their Raman-derived differences are as good or better than a patient’s FRAX score in distinguishing strong bone from weak bone,” Nyman said, referring to FRAX®, the online fracture risk assessment tool for diagnosing osteoporosis.
Nyman says clinical use of SORS may help navigate gray areas where a fracture might require partial or full joint replacement, if osteoporotic, but otherwise warrant fixation.
“Orthopaedic surgeons I talk to say the most difficult part about the fixation versus replacement decision is knowing whether the quality of the patient’s bone is sufficient to hold the bone screws,” he said. “We hope adding this information on fracture resistance will help in guiding these decisions.”