Catherine Watson, M.D., an assistant professor of obstetrics and gynecology at Vanderbilt University Medical Center, graduated from medical school in 2010, part of a cohort of gynecologic oncologists with a unique perch for witnessing a dramatic transition in endometrial cancer treatment.
As new attendings like Watson were pursuing their degrees, robust data on subtypes were not yet assembled and genetic sequencing of tumors was not readily available. For endometrial cancer, the gold standard was broad brush: surgery, possible radiation therapy and some chemotherapy choices for advanced disease.
“For the last few decades, everyone thought of endometrial cancer according to histology and classified it simply as high or low risk,” Watson said. “There were very limited treatment options, particularly for recurrent or advanced endometrial cancer. Now, studies on molecular features have brought to light the complexity and heterogeneity of these cancers. This gives us the opportunity to tailor treatment.”
Watson said she joined Vanderbilt largely for its resources that make personalized medicine – like genetic studies – a routine option for her patients.
“Clearly, the standard chemotherapy regimens don’t work as well as we want,” she said.
Complementing these resources are advancements in the surgical field.
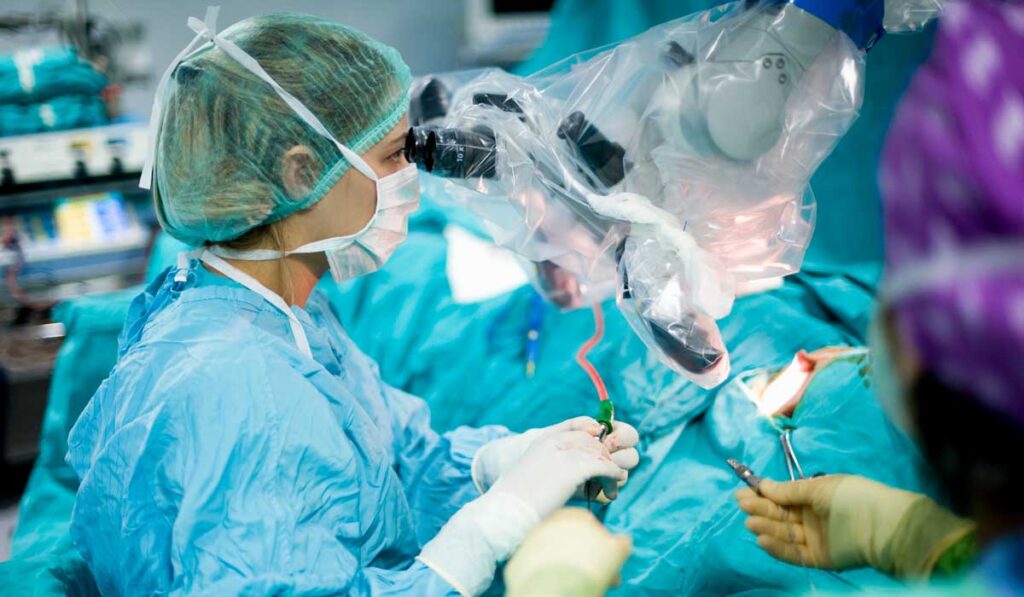
“Minimally invasive surgery is not inferior to open surgery for staging,” she said. “And sentinel node evaluation has become standard practice for many gynecologic oncologists. Both of these reduce morbidity considerably.”
Steep Survival Curve
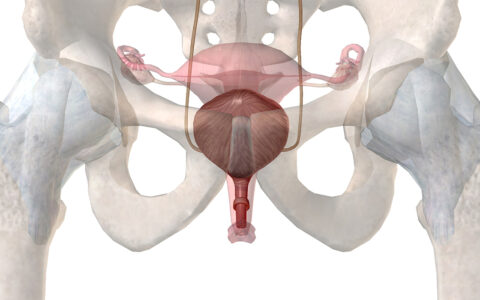
Uterine cancer is the most common female reproductive cancer. About 60,000 women in the U.S. have an endometrial carcinoma diagnosis each year, accounting for roughly 90 percent of uterine cancers. Most of these patients are postmenopausal. For the small percentage of cases occurring in young women, elevated BMI is often a driving factor.
“Clearly, the standard chemotherapy regimens don’t work as well as we want.”
Early treatment, before the cancer has spread outside the uterus, confers a five-year survival rate of 95 percent, but that figure drops to 69 percent for patients with regional metastasis and down to just 17 percent in those with distant metastasis.
Molecular Testing
In patients where endometrial cancer is hereditary, it is commonly attributed to germline mutations, mostly in mismatch repair genes. In 2013, The Cancer Genome Atlas reported a large scale, comprehensive and integrated genomic analysis of 373 endometrial carcinomas, focusing on molecular phenotypes within specific histologic grades. One of the key contributions of that study was the grouping of tumors into four distinct molecular subgroups.
“Recent studies suggest an important role for molecular characterization in these cancers, and in the near future, these characteristics could determine frontline treatment recommendations,” Watson said.
One example of this personalized approach is immunotherapy in the form of checkpoint inhibitors, which provide a huge advantage to patients with advanced or recurrent disease, whose tumors are microsatellite unstable or mismatch repair deficient. For patients with advanced or recurrent disease, Watson says they are seeing a doubling of progression-free survival rates using targeted immunotherapy with anti-angiogenics. This combination may potentially also benefits those without microsatellite unstable tumors.
Tangible Benefits to Patients – STAT
While these advancements may contribute to higher survival rates, Watson also notes that recent research on sentinel node biopsy is bringing immediate and tangible benefits to patients.
“Recent studies suggest an important role for molecular characterization in these cancers, and in the near future, these characteristics could determine frontline treatment recommendations”
“Especially for melanoma and breast cancer, there has been solid data to show that sentinel node biopsy was not inferior to a full nodal evaluation, but that had not been established in endometrial cancer until more recently,” Watson said. “With sentinel node biopsy, the risk of lymphedema is likely significantly diminished. This has a huge impact on patients’ quality of life, often for the rest of their lives.”
Minimally invasive robotic surgery is also gaining broader acceptance. Watson, who cut her surgical teeth on robotics, cites the lower blood loss and same- or next-day hospital discharge as key victories for patients and their medical teams.
Together with gene sequencing, she expects these developments to drive dramatically better prognoses for her patients.
“Personalized medicine is a hot topic in every field, especially in oncology right now, but I think endometrial cancer really demonstrates the potential benefit of it,” she said. “It’s still early, so it’s a very exciting time for me to start at Vanderbilt. I think in the next decade we’ll see huge changes in how we’re treating this disease.”