Diagnosing cardiac sarcoidosis, one manifestation of systemic sarcoidosis, is a thorny path for primary care physicians and general cardiologists. The condition may be viewed as too improbable to justify the confirmatory testing. Its symptoms – including shortness of breath, ventricular tachycardia, premature ventricular contractions and low ejection fraction – characterize myriad other conditions.
Dawn Pedrotty, M.D., a cardiologist and an assistant professor of medicine at Vanderbilt University Medical Center, says the mistaken perception of cardiac sarcoidosis as a rare disease is a big part of the problem. “The prevalence is really not known because of the high rate of missed diagnoses. Physicians often do not think to look for it, and thus it frequently just gets missed.”
Pedrotty recently joined Vanderbilt after starting the Cardiac Sarcoidosis Clinic at Mayo Clinic’s campus in Arizona. While at Mayo Clinic in Rochester, she completed a study on tissue archives that demonstrated a low rate of antemortem diagnosis of cardiac sarcoidosis. “The vast majority of decedents had been undiagnosed or misdiagnosed,” she explained. “Our first time diagnosing this disease cannot be on autopsy. We have to do better.”
To find ways of improving diagnosis, Pedrotty is working with electrophysiology colleagues at Vanderbilt to better understand what local inflammatory markers may be found in the hearts of patients with cardiac sarcoidosis.
Cardiac Sarcoidosis
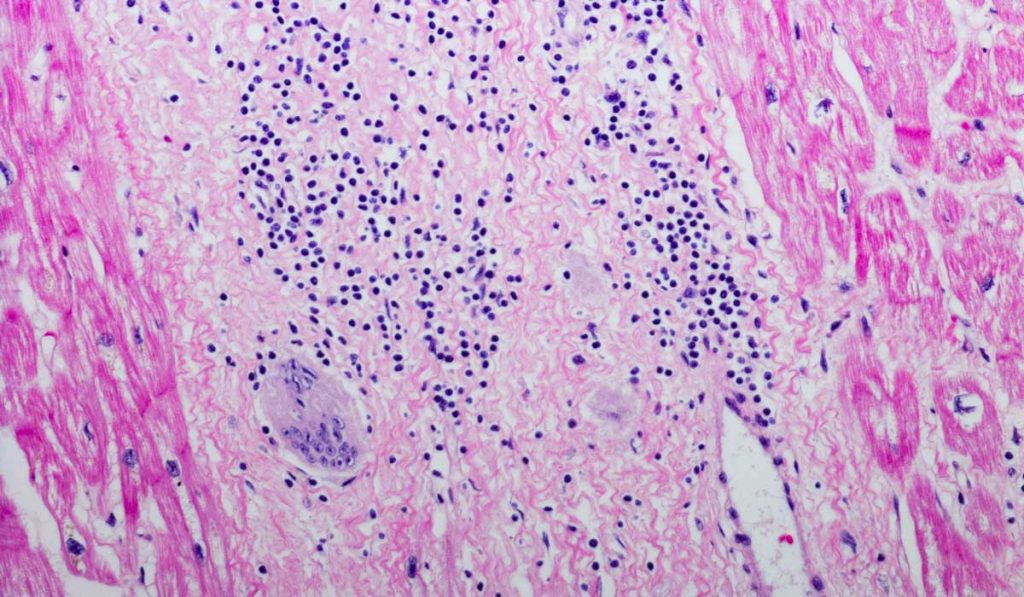
Sarcoidosis is a systemic granulomatous disease that can affect any organ. While the lungs are the most commonly affected, cardiac involvement portends a worse prognosis. Sudden cardiac death can be the first presentation of cardiac sarcoidosis.
“Our first time diagnosing this disease cannot be on autopsy. We have to do better.”
Early diagnosis, often prompted by the occurrence of arrhythmias, can lead to treatment with immunosuppressants, helping to reduce the arrhythmic burden and avoid scarring that can lead to heart failure. “It is similar to many chronic diseases,” Pedrotty said. “Some patients have only one episode or ‘flare’ of active inflammation, it’s treated, and they tend to do very well. In most of the cases, my patients come off immunosuppressants, at least intermittently.”
If not caught early, patients may develop worsening systolic heart failure and eventually require a heart transplant.
Challenges in Diagnosis
Discrepancies between antemortem and postmortem incidence rates of cardiac sarcoidosis is sounding an alarm that improved diagnostic methods and disease awareness are needed. While the disease is diagnosed in 2 to 5 percent of patients with systemic sarcoidosis, incidence of cardiac sarcoidosis detected postmortem is as high as 20 to 30 percent.
Pedrotty’s study documents the dramatic shortfalls in diagnosis. Her team looked at 36 cases of sarcoidosis identified in the autopsy archive. Of these, 12 were identified to have cardiac involvement, and only one carried a diagnosis of sarcoidosis. None had been correctly diagnosed with cardiac sarcoidosis.
“This was missed, even while two-thirds of the 12 patients had documented electrical abnormalities and other symptoms of cardiac sarcoidosis,” Pedrotty said. “We are battling lack of awareness regarding both the systemic disease as well as specific cardiac involvement.”
Toward Incisive Screening
Pedrotty says a PET scan is the best diagnostic tool, distinguishing between active and inactive disease. Since cardiac sarcoidosis is a “patchy” disease, Pedrotty says endomyocardial biopsies are accurate only about a quarter of the time.
“If a patient has a family history of sarcoidosis, refractory arrhythmias or a new diagnosis of non-ischemic cardiomyopathy, a cardiac MRI may be indicated,” she said. “If the results are suggestive of an infiltrative disease or inflammatory disease, then we do a cardiac PET scan to help guide therapy.”
“We are battling lack of awareness regarding both the systemic disease as well as specific cardiac involvement.”
Added Pedrotty, “If the disease is detected incidentally and is not active, we don’t treat it because of the significant risks of immunosuppression therapy. We do monitor those patients with an annual echo and EKG. If symptoms arise, we perform more advanced imaging.”
Seeking New Biomarkers
Pedrotty has now set her sights on identifying biomarkers to improve diagnosis.
“To assess trends in disease diagnosis, we are building a sarcoid database to connect several departments at Vanderbilt and link to other institutions’ databases, creating a larger repository of patient’s deidentified data,” she said. “We are also creating a tissue and blood bank to help us understand the pathophysiology of the disease and hopefully identify markers to enhance diagnosis.”