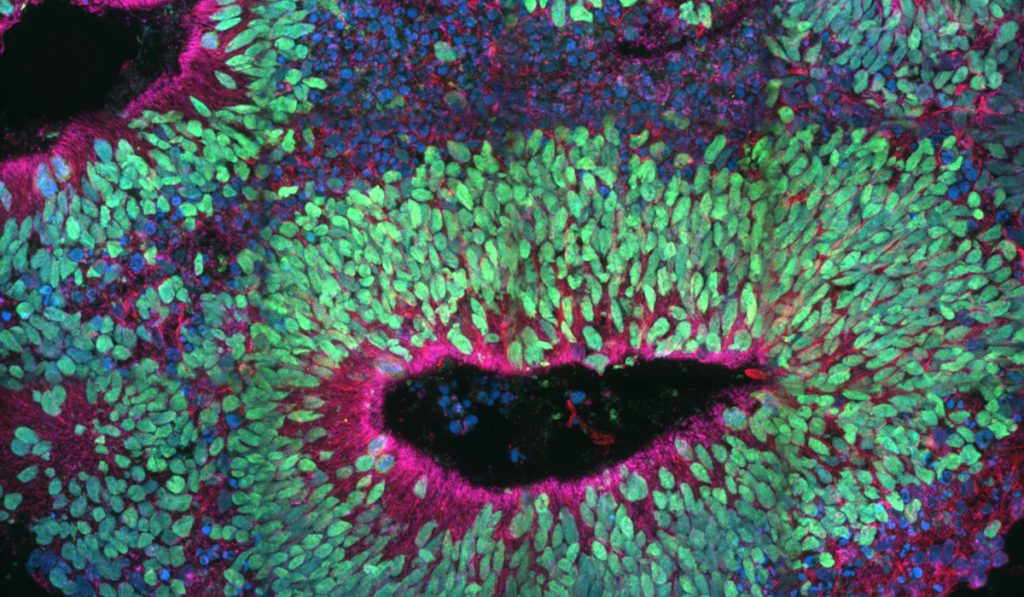
A multi-institutional research team is using models derived from knockout human induced pluripotent stem cells (hiPSCs) to study key factors and pathways involved in mitochondrial dynamics, apoptosis and cortical development.
Vivian Gama, Ph.D., an associate professor of cell and developmental biology at Vanderbilt University Medical Center, served as principal investigator on three studies (published in Cell Death and Disease, Stem Cell Reports and iScience) that helped uncover the roles of the pro-apoptotic protein MCL-1 and the BCL-2 family of proteins (specifically, BAX and BAK) in these critical developmental mechanisms.
“Elucidating how these proteins influence cortical development from hiPSCs deepens our knowledge of normal brain development and the etiology of neurodevelopmental diseases,” Gama said.
BAX/BAK in Human Cells
Understanding BAX and BAK function in human brain development has remained elusive for lack of precise model systems, Gama explained. To help remedy this, Gama and colleagues generated BAX/BAK double knockout hiPSCs, hiPSC-derived neural progenitor cells, neural rosettes and cerebral organoids. These in vitro models enabled study of early human brain development.
“Based on valuable mouse models, we hypothesized that BAX and BAK have critical functions during human development, including maintenance of homeostatic mitochondrial morphology. This is crucial for proper development of progenitors and neurons of the cortex,” Gama said. Affirming this was their finding that the BAX and BAK-deficient cells had abnormal mitochondrial morphology that gave rise to aberrant cortical structures.
“We knew that these proteins had a specific role in mouse development, but we wanted to learn what happens with human-derived systems to see if there is a similarity,” she said. “What we found was that these hiPSC-derived systems can be useful platforms to reveal novel functions of the apoptotic machinery in human neural development.”
Many Roles of MCL-1
Myeloid cell leukemia-1 (MCL-1) is a master regulator of apoptosis. Studies by the Gama laboratory provided evidence that MCL-1 also regulates mitochondrial dynamics. Variations in this regulatory machinery reduce cell viability and can cause severe neurological diseases.
“Maintaining mitochondrial morphology is crucial for homeostasis,” Gama said. “Scientists must understand the function of proteins associated with mitochondrial morphology, particularly in early development, in order to learn what goes wrong during disease. We’re working to increase our understanding of MCL-1’s role in the fundamental mitochondrial mechanisms that maintain pluripotency and self-renewal.”
“Elucidating how these proteins influence cortical development from hiPSCs deepens our knowledge of normal brain development and the etiology of neurodevelopmental diseases.”
In the Stem Cell Reports study, Gama’s team demonstrated that MCL-1 is a modulator of mitochondrial dynamics in hiPSCs and established the value of the stem cell model in studying the plasticity of the mitochondrial network during reprogramming and differentiation.
Key findings include:
- hiPSCs downregulate MCL-1 upon differentiation
- MCL-1 is induced during reprogramming, and its inhibition causes changes in the stem cell mitochondrial network
- Expression of MCL-1 at the mitochondrial matrix delays differentiation
- MCL-1 interacts with the protein DRP-1 and the protein OPA1
Unexpectedly, their study also uncovered a non-apoptotic function of MCL-1 in the maintenance of mitochondrial structure and stemness. Gama says this shift to a non-apoptotic function likely regulates the process of differentiation into progenitors and committed cells.
Potential Therapeutic Impact
Together, the studies are beginning to untangle key developmental processes in humans. “We are hoping that what we are learning about dysregulated mitochondrial dynamics at the cellular level – through these hiPSC-derived model systems – will lead to novel therapeutics in the future,” Gama said.
The findings could have life-altering implications. “As an example, there are children with rare mitochondrial diseases who currently don’t survive past five years of life. Understanding the function of mitochondrial morphology during development may help these patients in the future.”