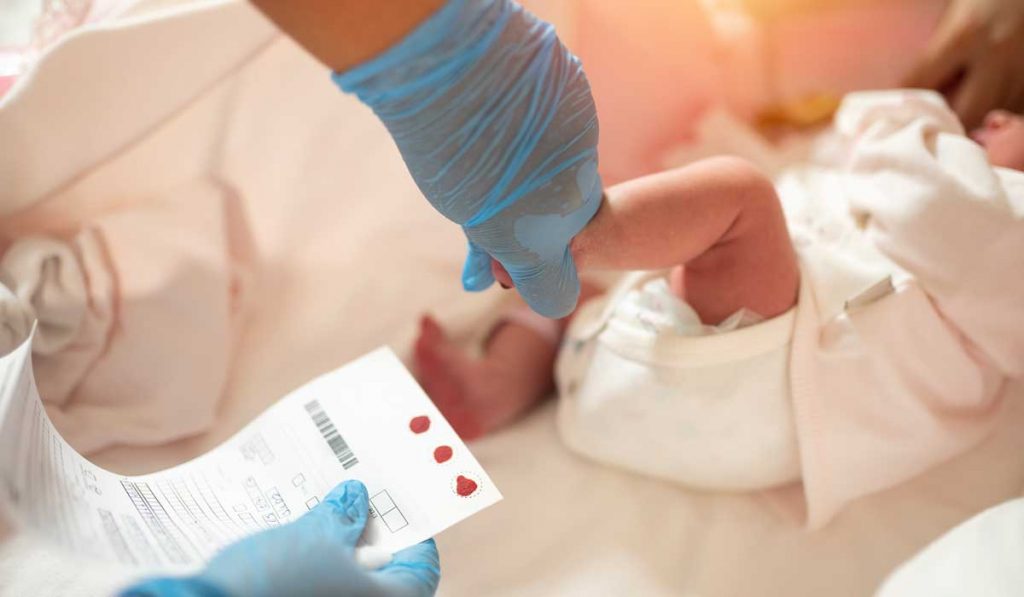
At Monroe Carell Jr. Children’s Hospital at Vanderbilt, the first child with methylmalonic acidemia (MMA) has been treated with hLB-001 gene therapy, which uses an adeno-associated virus (AAV) vector to correct a genetic defect.
Today, AAVs are used in vaccines against Ebola, Zika, malaria and even influenza. AAVs are also used in many trials for curing both rare and common diseases, by delivering new genetic instructions to cells that are currently producing and reproducing harmful proteins.
Thomas M. Morgan, M.D., an associate professor of pediatrics and medical genetics at Vanderbilt University Medical Center, is a principal investigator on the SUNRISE trial, which will be enrolling seven additional children with MMA at seven study sites to test the AAV approach.
“The fact that this is a phase 1/2 trial reflects the confidence we have in the safety of this therapy.”
“Children with MMA can’t metabolize proteins well because they don’t manufacture mutase – one of the necessary enzymes. We are doing a one-time infusion to deliver the gene they are missing into their liver cells,” Morgan said. “If we are successful, the liver will have new, lifetime instructions to make mutase. In time, the child could have a normal diet for the first time in their life. Most importantly, we hope they will avoid severe illness and irreversible organ damage.”
Amino Acid Balancing Act
The culprit in MMA is a mutation in the gene encoding methylmalonyl-CoA mutase (MMUT). Without a functioning mutase enzyme, a person cannot ingest four common amino acids – valine, methionine, isoleucine or threonine – without a hazardous build-up of methylmalonic acid in the bloodstream that threatens the health of multiple organs.
In Tennessee, MMA is one of 71 genetic abnormalities in a newborns’ heel stick screening. MMA affects one in 50,000 to 100,000 live births. Symptoms include vomiting, dehydration, weak muscle tone, lethargy and failure to thrive.
“We are doing a one-time infusion to deliver the gene they are missing into their liver cells.”
“Once diagnosed, we give these children just enough natural protein to live and to , and then we try to optimize the level of those four amino acids through a special formula that has everything else in it but those four amino acids,” Morgan said.
The severity of MMA is highly variable, but Morgan says for most patients this restrictive diet does not protect them from getting sick or potentially needing a liver or combined kidney and liver transplant later in life, so other approaches are desperately needed.
hLB-001 Gene Therapy
In the SUNRISE trial, the hLB-001 AAVs are engineered to be tropic to the liver, so that they don’t permeate the body, potentially destroying tumor suppressant cells. “Cleverly,” Morgan says, “a string of nucleotides guides the DNA package to the nucleus of the hepatocytes, and deposits it next to albumin, the most abundant protein of the liver. This proximity is key, because albumin is what drives the production of mutase.”
To determine the extent of penetration into the liver cells’ DNA, researchers added a tag to the albumin protein so it creates a biomarker, “albumin 2A.” Researchers will also look for a reduction in methylmalonic acid in the blood and check for liver inflammation and other immune reactions.
“The fact that this is a phase 1/2 trial reflects the confidence we have in the safety of this therapy,” Morgan said. “If everything goes as expected, I anticipate an extension of the trial and a broadening of inclusion criteria, particularly to older patients.”
A Beneficial Cascade
Morgan sees AAV gene therapy as being in a proof-of-concept stage, with rare disease patients paving the way, optimizing the technology for broader use. “We have very small patient populations with very rare and severe diseases. These are the people who are going to take whatever risks there are, but potentially get the earliest benefits.”
The large burden of hope invested in this technology is sobering, given its complexity and infancy as a working therapy. Morgan says excitement is warranted. “Today, over 7,500 genetic diseases have been identified and catalogued. Most of that has happened during my career,” Morgan said. “Imagine the impact this could have on hundreds of thousands of lives.”