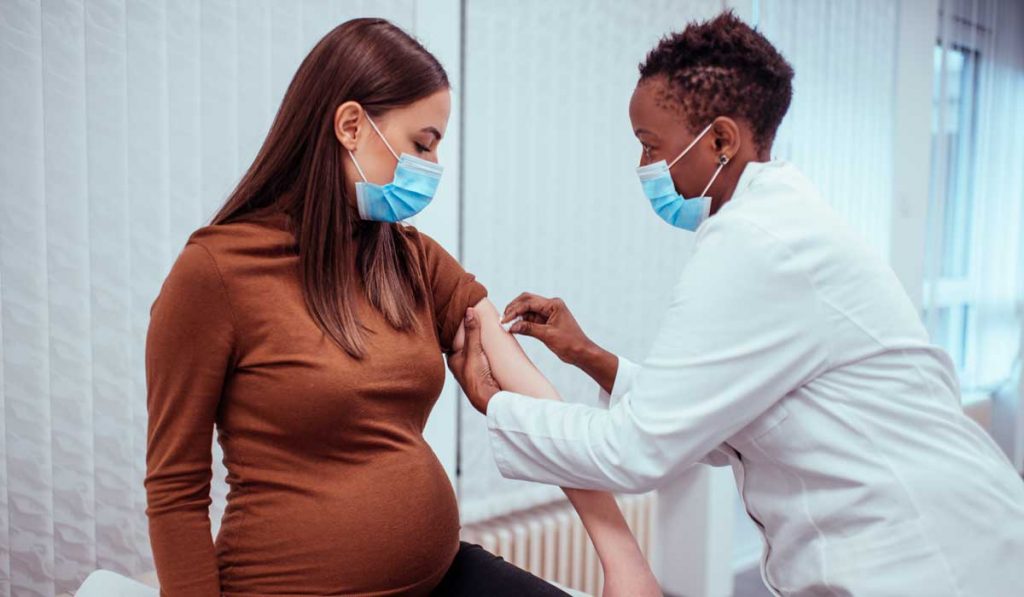
A growing number of women are receiving a COVID-19 vaccine while pregnant. Although limited, safety data for this specialty population are also growing. One recent study following pregnant individuals who received the Pfizer or Moderna vaccine found no increased risk of adverse outcomes compared to baseline risks among 827 completed pregnancies.
“The ongoing data that we’ve seen from pregnant women receiving the vaccine continues to reassure us,” said Jennifer Thompson, M.D., an associate professor of obstetrics and gynecology and a specialist in high-risk pregnancy at Vanderbilt University Medical Center.
Higher Baseline Risk
Thompson emphasizes pregnant women are at increased risk for severe COVID-19 illness. “They have increased risk of hospitalization, need for ICU, mechanical ventilation, and a slightly increased risk of death compared to non-pregnant patients,” she said. At least 97 pregnant women have died from COVID-19 in the U.S.
The CDC also reports pregnant women with COVID-19 “might be at increased risk of adverse pregnancy outcomes, such as preterm birth.”
Said Thompson, “Because of that increased risk, women who are pregnant are considered a higher risk group. That’s why our organizations including the CDC, the American College of Obstetricians and Gynecologists, and the Society for Maternal-Fetal Medicine recommend that the COVID-19 vaccine should not be withheld from pregnant individuals.”
Cause for Concern?
In short, risk of COVID-19 illness far outweighs risk of vaccination, Thompson says. Developmental and reproductive toxicity studies in rodents showed no adverse effects from the three vaccines approved for emergency use in the U.S.
“The ongoing data that we’ve seen from pregnant women receiving the vaccine continues to reassure us.”
Still, the American College of Obstetricians and Gynecologists recommends pregnant persons stick to the Pfizer or Moderna vaccines, after six cases of cerebral venous sinus thrombosis were reported among recipients of the Johnson and Johnson formulation. Such announcements may rattle confidence among pregnant individuals, Thompson says. She notes public health research is ongoing – and critical – to measure vaccine confidence and identify influencers among pregnant women and mothers.
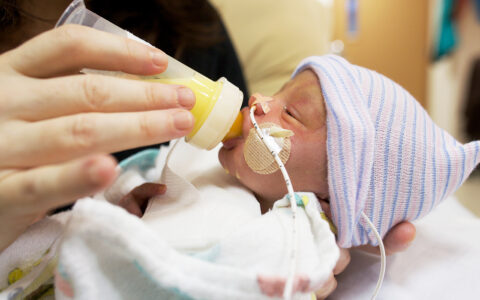
Potential Benefits for Neonates
New data suggest pregnant persons who receive a COVID-19 vaccine are in fact protecting their babies against SARS-CoV-2 infection. Maternal IgG antibodies to SARS-CoV-2 transfer across the placenta and are also found in cord blood.
One prospective study among 131 reproductive-age women found SARS-CoV-2 antibody titers were significantly higher across pregnant, lactating and non-lactating women who were immunized as compared to women with prior SARS-CoV-2 infection. The study also confirmed robust IgG transfer via the placenta and breast milk. While one woman experienced spontaneous preterm labor 17 days after receiving her first vaccine dose, this was at 35 weeks’ gestation and the authors noted no safety issues for pregnant and breastfeeding women.
Additional Data Gathering
Over 86,000 pregnant persons have already received a COVID-19 vaccine. The CDC is encouraging them to participate in the V-safe COVID-19 Vaccine Pregnancy Registry to help better understand reproductive effects. An international registry seeks similar outcome data.
Vaccine manufacturers also continue to collect prospective and longitudinal data. Pfizer is currently enrolling 4,000 pregnant women in a new trial; Johnson and Johnson has long-term plans to conduct safety and immunogenicity trials in infants and children.