The gut microbiome is a key link between genetic susceptibility for inflammatory bowel disease (IBD) and the onset and progression of the condition. Although the mutually beneficial relationship between humans and microbes residing in the gut is clear, how their functions influence one another is still poorly understood.
Fang Yan, M.D., a research professor of pediatrics at Monroe Carell Jr. Children’s Hospital at Vanderbilt, and colleagues are using p40, a protein secreted by the commonly used probiotic Lactobacillus rhamnosus GG (LGG), as a tool to investigate the relationship between intestinal epithelial cells and beneficial gut microbes.
A recent study published in Cellular and Molecular Gastroenterology and Hepatology found that p40 supplementation in early life induces long-lasting effects on prevention of IBD in adulthood.
“Multiple studies have shown that an abnormal gut microbiome correlates with increased susceptibility to IBD. However, the mechanisms by which the microbiome transmits health-promoting signals and imprints them onto intestinal ecosystems remain largely unknown,” Yan said. “Our research seeks to fill in the gaps.”
Impact of p40 on Inflammation
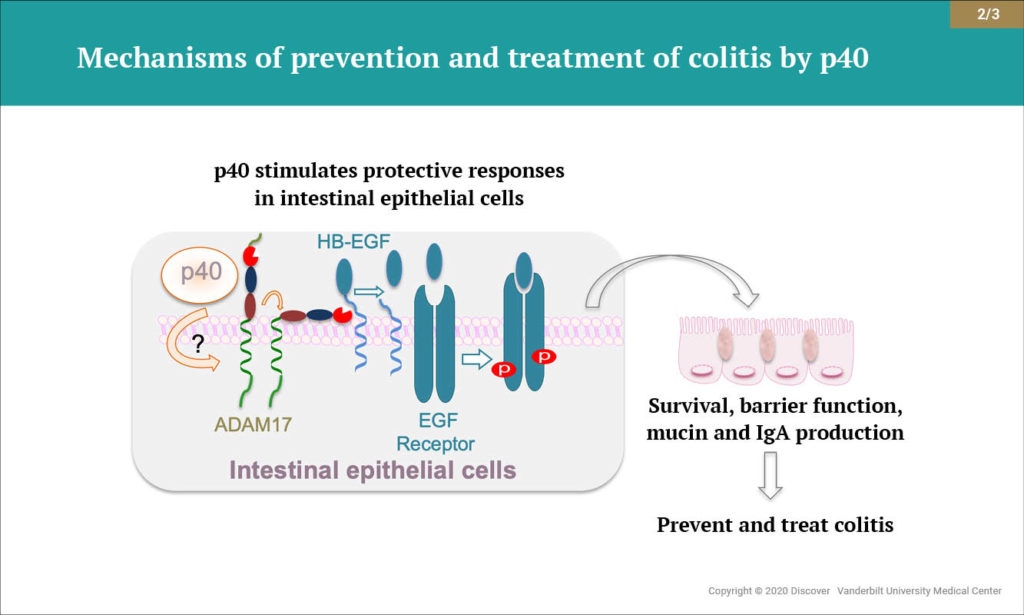
Yan and colleagues were the first to identify a novel mechanism of communication by which LGG promotes the survival of intestinal epithelial cells. In subsequent studies, they showed that p40 promotes intestinal epithelial homeostasis through specific signaling pathways. Notably, they found that p40 activates epidermal growth factor receptor, promoting normal intestinal development, and upregulates production of immunoglobulin A, a key defense against intestinal disease.
The researchers expanded their scope and demonstrated that therapeutic delivery of p40 to the colon of mice using a hydrogel bead system reduced intestinal inflammation. Further, they found that p40 supplementation in early life protected against intestinal inflammation and injury in adult mice. The new study builds on this research.
Burden of Childhood IBD
Yan’s findings could have a significant impact. Approximately 1.6 million Americans currently have IBD, and as many as 70,000 new cases are diagnosed in the U.S. each year. Up to a quarter of patients with IBD develop intestinal inflammation during childhood and adolescence, and young patients have a more severe disease course than adults, Yan noted.
“There’s a significant need for approaches which could promote the establishment of the gastrointestinal tract during development in pediatric patients with IBD,” Yan said. She added that p40 supplementation in early life might provide nutritional support and increase mucosal defense capacity, serving as a preventative strategy for individuals at high risk of developing IBD.
A Symbiotic Relationship
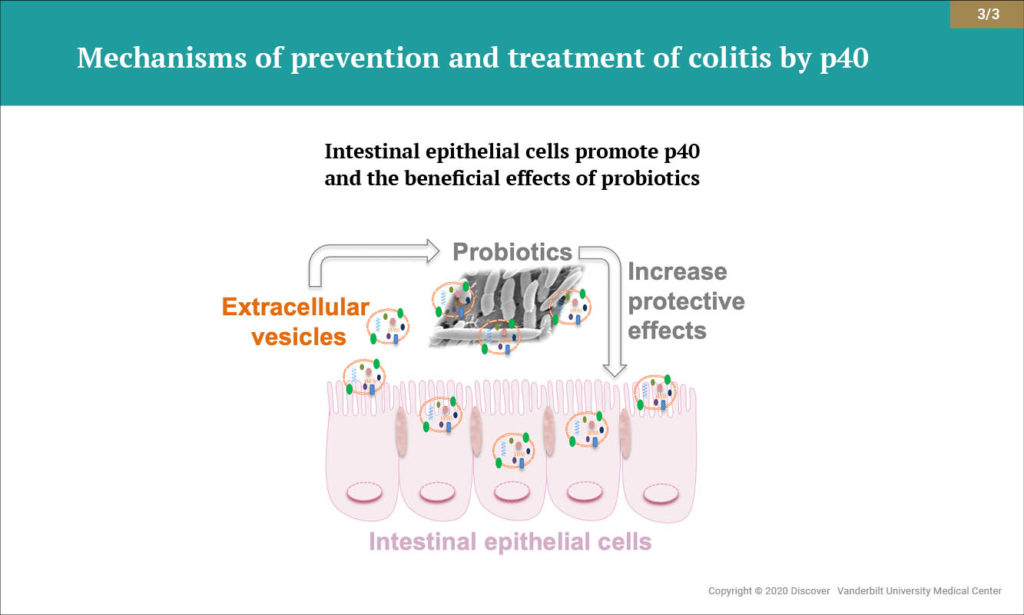
Increasing evidence indicates that extracellular vesicles (EVs) secreted by the surfaces of intestinal epithelial cells are important messengers for maintaining intestinal homeostasis. Research from Yan and colleagues has revealed a previously unrecognized mechanism by which EVs derived from intestinal epithelial cells reinforce microbiota functions that benefit the host.
The authors found that p40 production was upregulated when LGG was cultured with content from the colonic lumen of healthy mice, but not with content from mice with intestinal inflammation. Culturing LGG specifically in the presence of EVs from intestinal epithelial cells promoted p40 synthesis and secretion. The findings demonstrate that EVs released from intestinal epithelial cells reinforce p40 production by LGG, enhancing the bacteria’s protective effect against colitis.
Toward IBD Therapeutics
Yan says the next step is to find ways to increase the regulatory effects of EVs on LGG and p40 function. She and her colleagues hold U.S. patents for novel compounds they plan to test as potential enhancers.
“Our results provide a mechanistic understanding of how the gut microbiota contribute to benefiting host health,” Yan said. “Now we hope to develop microbiota-based therapies for maintaining intestinal health and for preventing or treating Crohn’s, colitis and other crippling diseases caused by intestinal inflammation.”