A new laboratory technique is allowing researchers to manipulate and reconstruct human islets in 3D, enabling a deeper understanding of signaling pathways central to diabetes. A team of islet biologists, physiologists and biomedical engineers have published technical details and a proof-of-concept application in JCI Insight.
“We have provided all the experimental details and our protocol so others can make the media and create pseudoislets in their own laboratories,” said senior author Marcela Brissova, Ph.D., director of the Islet Procurement and Analysis Core at Vanderbilt University Medical Center. “We hope our technique will help advance understanding of intracellular dynamics that occur in diabetes.”
“We hope our technique will help advance understanding of intracellular dynamics that occur in diabetes.”
Islet Challenges
Pancreatic islets contain myriad cell types: β, α, and δ cells that control glucose homeostasis; immune cells; plus nerve fibers and endothelial cells that support islet structure and function. Dysfunction of these islet cells is a primary component of all forms of diabetes.
A major challenge has been how to study cell-specific signaling pathways within the context of their 3D arrangement – particularly for cells at the center of the islet “sphere.”
“The 3D islet architecture, while essential for function, presents experimental challenges for mechanistic studies,” the authors wrote.
Rebuilding Islets
Brissova and colleagues sought a system that would enable them to genetically manipulate specific cells within islets and analyze the results.
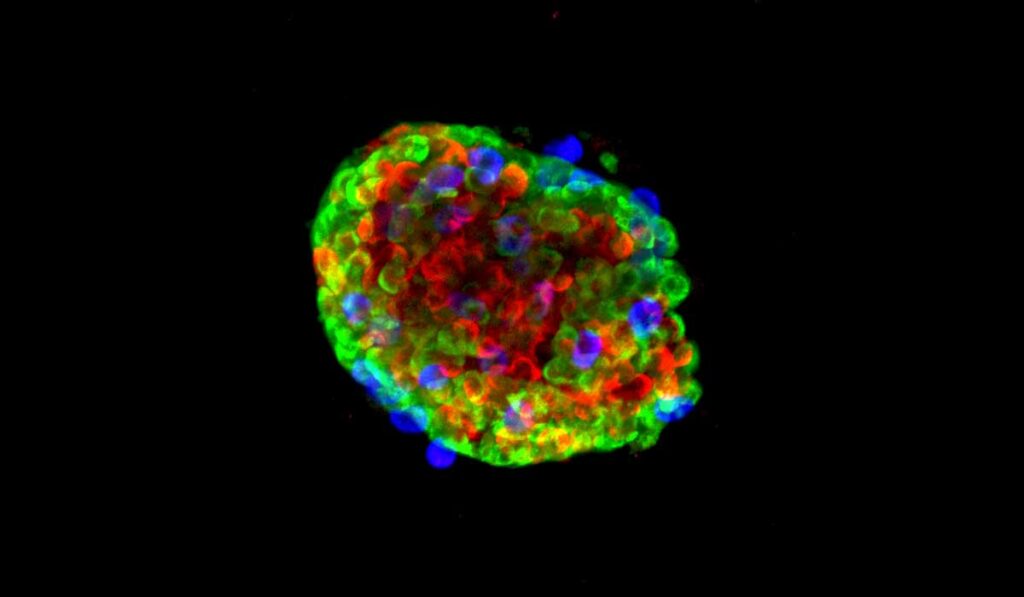
Their newly developed protocol starts by separating pancreatic islets into single cells. The researchers then perform virus-mediated manipulations, finally combining the cells back together again into a “pseudoislet.” The reaggregated pseudoislets, which take less than a week to form, are representative of native human islets and reveal functional consequences of any manipulations.
“Using primary human islets, we developed a pseudoislet system that resembles native human islets in morphology, cellular composition, cell identity, and dynamic insulin and glucagon secretion,” the authors wrote.
In the proof-of-concept, the team added a unique microfluidic device to study insulin and glucagon secretion and intracellular signaling dynamics. They activated a G-protein-coupled receptor signaling pathway in pseudoislets and showed distinct differences in hormone secretion between β and α islet cells, accompanied by dynamic changes in intracellular calcium.
Roads to Success
Brissova and her colleagues have been perfecting laboratory conditions for pseudoislets for several years. “A lot of things in science happen serendipitously, and this was one of those,” she said. “We tried and failed many times, and basically it came down to the media we used for our cells.”
Now, the team is focused on developing pseudoislets that represent specific disease states, such as type 1 diabetes in children.
“It’ll be really exciting to see how it will branch out into different areas of islet biology.”
“It’ll be really exciting to see how it will branch out into different areas of islet biology,” Brissova said. “I believe this system will help us better translate information from mouse model studies, test effects of genes identified by human type 1 and type 2 diabetes studies, and better study mechanisms of future therapeutics.”