New research published in Nature suggests that epigenetic therapies targeting myeloid-derived suppressor cells (MDSCs) could effectively prevent formation of cancer-receptive niches – if they are used with a precise approach to timing, dosage and combination of agents.
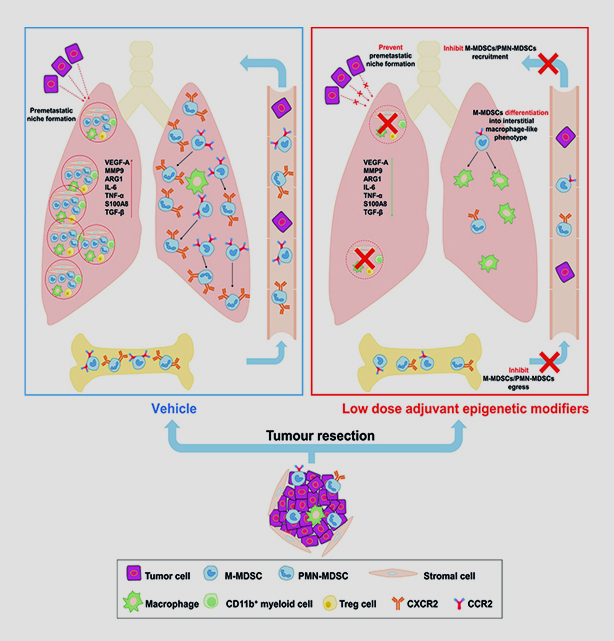
Myeloid cells aid the spread of lung, breast and esophageal cancer by forming MDSCs that prime potential metastatic sites and promote metastasis. The new mechanistic study found that at the end of six weeks, mice with MDSC-inhibiting interventions survived and were cancer-free, while untreated mice died after just two weeks.
“Our research showed that MDSCs play a vital role in enabling metastasis and that we can effectively interfere with their metastasis-promoting mechanism,” said Young Kim, M.D., co-leader of the translational research and interventional oncology research program at Vanderbilt University Medical Center and co-author of the study. “It is reasonable to envision that this therapy can prevent metastasis in patients in stage one or two cancer, as adjuvant therapy after surgical resection.”
Immune Ally Turns Enemy
One reason many cancer patients metastasize several years after even a clean resection of their tumor is that residual cells find an ally in the patient’s own immune system, Kim says.
“It’s not the tumor cell itself that is the big problem. Our own myeloid cells are recruited to assist in the cancer’s enterprise to spread,” he said. ”Imagine a colorectal cancer cell that has been adapted to grow in tissue in the colon lining. It would be difficult for that colorectal cancer cell to survive in a lung or liver environment. The MDSCs are one of the keys to allowing the metastatic cells to thrive in a new site.”
“The MDSCs are one of the keys to allowing the metastatic cells to thrive in a new site.”
Trans-grafted Mice Elude Metastasis
Kim and collaborators at multiple institutions including Johns Hopkins University focused on controlling the metastatic process in mice that had resected lung tumors but no metastasis. One set of experiments showed that the donor MDSCs from a tumor-bearing mouse promoted metastasis in another. A second showed that suppressing epigenetic signaling in the exogenously transferred MDSCs eliminated metastasis in host mice.
The successful treatment included low-dose DNA methyltransferase and histone deacetylase inhibitors (5-azacytidine and entinostat) that targeted MDSCs and changed their function into a more interstitial macrophage-like phenotype. The researchers further suppressed lung metastasis with antibodies that target MDSC surface molecules used to promote tissue trafficking (i.e., downregulating CCR2).
“By going back and forth between two separate models, we could control what kind of MDSCs are introduced back into the second [group of] mice and actually show that our modifications of this signaling were responsible for the prevention,” Kim said.
Broader Significance
While the study examined epigenetic signaling only in lung cancer metastasis, Kim says the therapeutic strategy may have broader applicability. Next, Kim and his team are looking at applying epigenetic therapy to head and neck cancer and to breast cancer.
”Since we are targeting immune cells, we expect applications to extend to many types of tumors that have high potential to metastasize. The key would be tailoring each therapy regimen to the epigenetic signaling of that particular cancer,” Kim said.