While there are an exciting assortment of model systems for researching clear cell renal carcinoma (ccRCC), the flood of options can pose a challenge in selecting the one most appropriate for a study.
“It has been hard for people to know what all the available models are and which to choose,” said Kimryn Rathmell, M.D., an expert on kidney cancer and interim chair of the Department of Medicine at Vanderbilt University Medical Center. Rathmell says her laboratory is frequently contacted by investigators seeking guidance regarding RCC model systems.
In a recent review article in Oncogene, Rathmell and co-authors have summarized the available model systems for ccRCC as well as those in development, highlighting the strengths and limitations of each.
“The data are out there but have not been compiled nicely, until now. That was one of the most valuable things of this review, being able to point people in the right direction,” Rathmell said.
Characteristics of ccRCC
A ccRCC is characterized by loss or mutation of the von Hippel Lindau (VHL) gene located on chromosome 3p. VHL encodes the oxygen-sensing mediator protein VHL. In its absence, cellular programs regulated by oxygen levels become dysregulated, including glycolysis, angiogenesis and lipolysis, leading to nutrient and oxygen imbalances in the tumor microenvironment.
Rathmell explains that while the vast majority of ccRCCs exhibit a characteristic loss of both copies of VHL, the secondary and tertiary mutations will differ, resulting in another characteristic feature of ccRCC – significant intratumoral heterogeneity. “There is some genomic instability that is causing them to have an opportunity to acquire multiple different paths to transformation.”
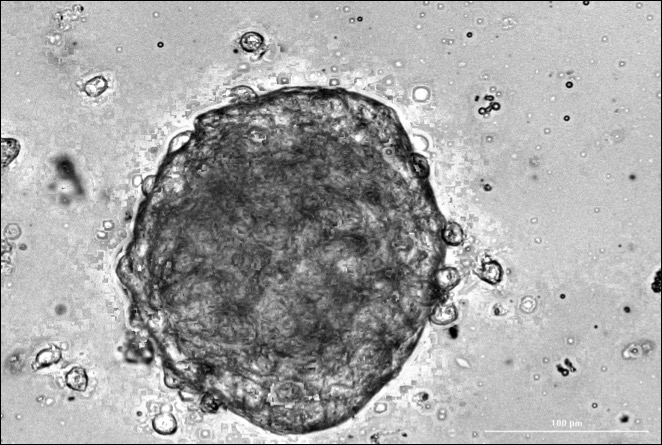
ccRCC Model Systems
Model systems include traditional cell culture systems, 3D organoids, cell-line-derived (CDX) or patient-derived (PDX) xenografts, and genetically engineered mouse models. Rathmell emphasizes that the choice of model system must be driven by the research question being asked.
”The model systems each have different specific components of kidney cancer that they address individually,” Rathmell said. “For fundamental questions related to genetics and molecular biology that’s housed within a cell, cell culture is great. If you need architecture and multicellularity, you need to be working in mice. If you need to be looking at the whole breadth of human cancers, you need something that comes from primary patient samples.”
“You can gain a little bit of useful information from each of the model systems to complete a comprehensive study.”
“You can gain a little bit of useful information from each of the model systems to complete a comprehensive study,” said Melissa Wolf, first author on the review and a graduate student at Vanderbilt working in the Rathmell laboratory.
Future Research
Standard first line treatment for metastatic ccRCC is an antiangiogenic agent in combination with an immunotherapy. While immunotherapies are remarkably effective at treating ccRCC, Rathmell says that it’s not clear why.
“Some of the features that drive the heterogeneity probably are what make it susceptible to immunotherapy drugs. But really, we don’t understand very well why this cancer more so than others can respond to the checkpoint inhibitors.”
Rathmell and Wolf emphasize that, in the future, it will be important to develop sophisticated models of ccRCC that more accurately mimic the tumor microenvironment.
Wolf is working to develop a novel patient-derived 3D organoid system for ccRCC, with the goal of capturing the full scope of tumor heterogeneity. “Successful models that recapitulate the tumor environment will help us get a better look at the tumor and immune cell cross-talk, and how that contributes to the development and spread of the disease,” she said.