The Kidney Project team is making gains in one of the greatest challenges to their goal of miniaturizing a bioartificial kidney for implantation: developing a functional bioreactor.
Until recently, project leaders William H. Fissell, M.D., associate professor of medicine and biomedical engineering at Vanderbilt University Medical Center, and Shuvo Roy, Ph.D., professor of bioengineering at University of California San Francisco, have been hindered by one particularly thorny obstacle: how to grow renal tubule cells in vitro that look and behave like they do in the body.
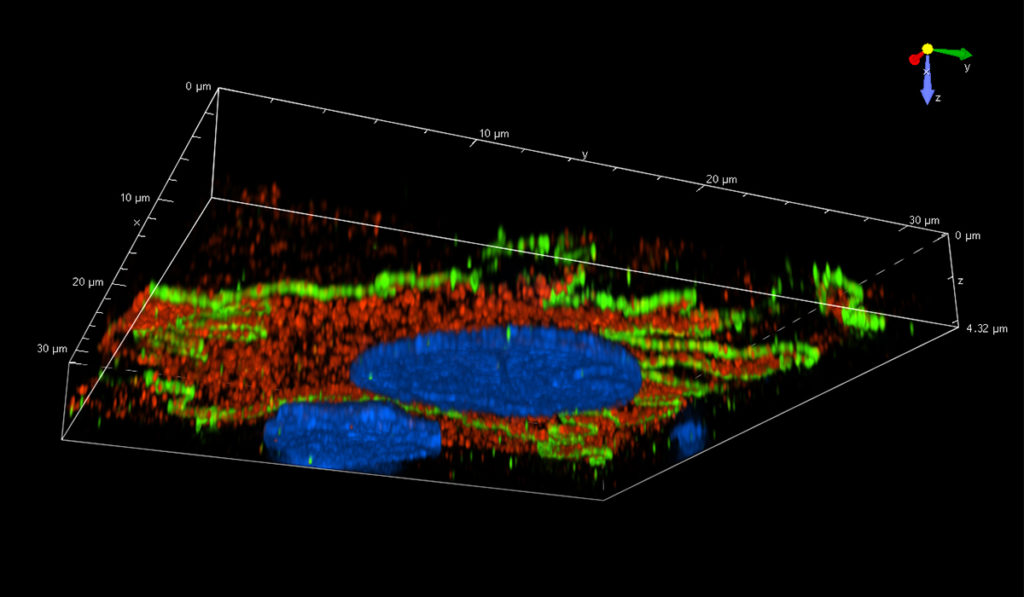
Now, using a combination of a TGF-ß inhibitor and metformin, the team has found a means of mitigating the stress of cell culture. “This achievement overcomes a major hurdle limiting the development of a bioreactor of cultured cells for renal replacement therapy that encompasses not only endocrine and metabolic functions but also transport and excretion,” the research team wrote in Tissue Engineering.
A Key Barrier to Implant
Fissell and Roy are the inventors of a new membrane technology for renal failure that could replace dialysis, inspired by the seminal artificial kidney development work done by Fissell’s mentor H. David Humes, M.D., professor at the University of Michigan. This earlier discovery was a major step toward the two’s vision of a fully implantable, “soda can” size artificial kidney.
However, Fissell says many were skeptical of their ultimate success because they would need to first grow functional kidney cells outside the body. “The reality is when you grow cells in a plastic dish, cells that normally look like Bart Simpson’s head look like fried eggs,” Fissell said. “These stressed cells don’t function well, so we had to identify the factors stressing the cells, and it’s a long list of possibilities.”
“We had to identify the factors stressing the cells, and it’s a long list of possibilities.”
Shaping Renal Cells
One of the cues Fissell tested was the stiffness of the surfaces used for growing the cells. Kidney cells grew differently on gels with stiffnesses matching healthy kidney tissue. Fissell and Roy first looked for solutions in soft hydrogel scaffolds substrate.
“These three-dimensional soft scaffolds are great for a lab experiment but not for our needs, so we asked ourselves if there was a drug that could provide the same effect.” The cytokine TGF-b is known to be involved in the cellular response to scarring, so Fissell and Roy tested the effect of blocking TGF-b.
They added metformin, a drug that modifies how cells use available energy sources, to the cell culture solution. The cells responded and maintained the repertoire of proteins similar to what they have when immersed in fluids flowing through the kidney. “When we grew cells on this stiff scaffold, but gave them an inhibitor, they looked a lot more like the cells grown on a soft scaffold,” Fissell said.
“When we grew cells on this stiff scaffold, but gave them an inhibitor, they looked a lot more like the cells grown on a soft scaffold.”
Proof-of-Concept
Nine weeks after in vitro seeding, the team observed a 4.5-fold increase in apicobasal transport in primary renal tubule cells when TGF-ß inhibitor or metformin was added alone and an 8.5-fold increase when both were used together. “We’ve now finally shown that, yes, it’s a challenge to get these cells to differentiate in a conventional lab dish, but you can do it,” Fissell said. “This problem was one of the fundamental objections to our goal that we’ve been parrying all these years.”
To move forward in his work, Fissell faces funding challenges that have become a familiar refrain throughout this project. “We’ve completed the germinal work supported by organizations like the NIH, but the time to commercialization is still distant for venture capital to fund,” he said. “We have been extraordinarily fortunate to have had philanthropic support from families who have been touched by kidney disease and want something better for tomorrow.”