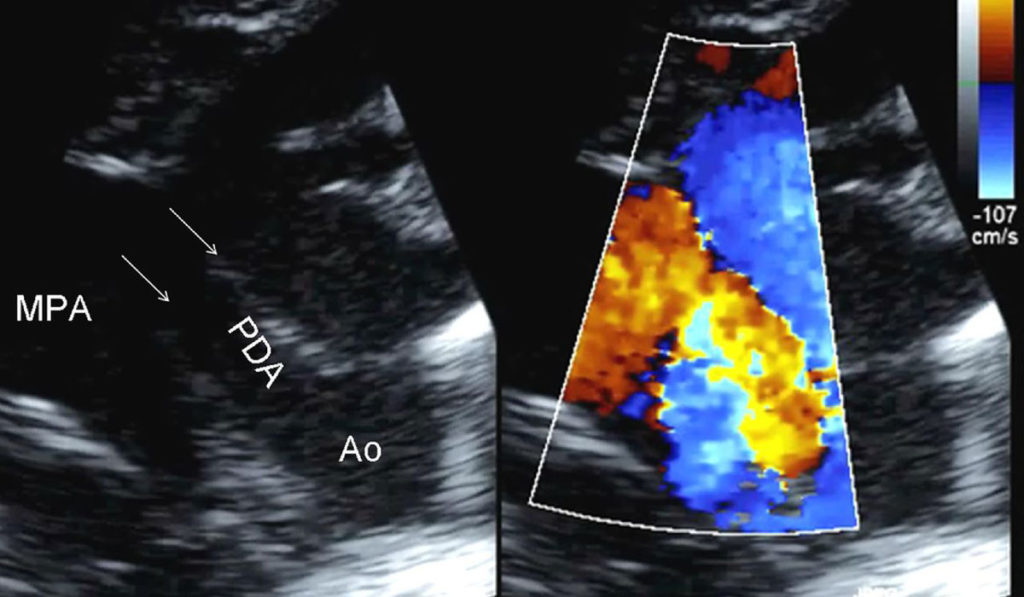
Treatment of patent ductus arteriosus (PDA) is controversial and plagued by high practice variation, with numerous underlying questions. Which babies are better candidates for medication, ligation or no intervention? How long do you wait for spontaneous vessel closure? How should PDA size, age at birth, and weight factor into treatment decisions?
“The consternation over which babies and which treatment to use can turn management decisions into a hand-wringing exercise,” said Jeff Reese, M.D., a neonatologist at Vanderbilt University Medical Center. Reese believes that over the past 20 years the treatment pendulum has swung too far away from medication (COX inhibitors) in favor of conservative management and waiting for a PDA to close on its own. He and colleagues published a recent article in the Journal of Perinatology advising a more balanced approach.
“The consternation over which babies and which treatment to use can turn management decisions into a hand-wringing exercise.”
“Each decision carries risks, and these are magnified the younger, smaller and more fragile the infant is,” Reese said. Medication intended to constrict the PDA also affects the other vasculature, but waiting for the PDA to close on its may raise the odds of bronchopulmonary dysplasia and adverse consequences for the brain, heart, gut and kidney.
Decision Complications
The age of preterm viability now hovers around 22 weeks; infants born at 23 to 24 weeks gestation have a 70 percent chance of PDA versus a 59 percent chance among those born at 25 to 28 weeks. “Because the viability envelope is being pushed, more vulnerable babies are out there. We are in the midst of a swinging pendulum and a medical quandary in defining better treatment,” Reese said.
The challenge begins with diagnostic vagaries, with only broad echo categories for characterizing a PDA, Reese says. Once the PDA is characterized, the choice to wait or administer COX inhibitors is influenced by other factors, including the fact that up to 40 percent of babies do not respond to the medications.
Added to the decision matrix is the option of surgical ligation to close the PDA, and in some centers, including Vanderbilt, the ability to block the ductus using less-invasive approaches and deployment of new catheter-based occlusion devices.
On the Brink of Precision Therapies
The debate may ultimately be settled by new therapies. Vanderbilt developmental biologist Elaine Shelton, Ph.D. is collaborating with Reese and others to identify which vessels require intervention, and to define genetic differentiators that would enable a personalized medicine approach to PDA therapy. In addition, Shelton’s lab is focused on developing new, selective PDA drugs that specifically and more efficaciously target the ductus, while leaving other vascular beds alone.
Using human ductus cell lines, transcriptome analysis, and high throughput screens, the researchers are beginning to identify novel PDA drug targets, expanding the current therapeutic repertoire beyond the COX signaling pathway. One promising target, an ATP-sensitive potassium ion channel, or KATP channel, may fit the bill.
Tissue-specific KATP channel isoforms are found throughout the body, but the vascular form of the channel is significantly enriched in the ductus compared to other vascular beds, Shelton explained. “This allows for the possibility of targeting them with a ‘Goldilocks’ dose of the drug – one that would have just the right effect on the ductus, but would not impact the other tissues.”
The team is now developing small molecule inhibitors to precisely target vascular KATP channels. “Once we find a compound that specifically targets only vascular KATP channels, we can develop a medication that constricts the ductus but leaves everything else alone.”
More Promising Research
Shelton is also studying the basic molecular mechanisms of what keeps the ductus open in utero and what helps it close after birth. Her team found that maturity of the muscle cell, oxygen-sensing mechanisms, biomechanical factors, and certain mutations in the KAPT ion channel all impact closure.
Additionally, Shelton is analyzing genetic characteristics of infants with and without PDAs to identify who is at greater risk for PDA. “It is not currently possible for clinicians to accurately predict which PDAs will close spontaneously and which will require intervention,” she said, noting a genotype of the infant could inform treatment decisions in the future.
“It’s only a matter of time before we can develop new medications and personalized treatment to hopefully put an end to the controversy.”
“We are looking, on a gene level, on a protein level, for what sets the ductus apart from all the other vessels in the body and what differentiates one infant’s susceptibility to PDA from another’s. It’s only a matter of time before we can develop new medications and personalized treatment to hopefully put an end to the controversy,” Shelton said.