The molecular underpinnings of the most common complication of preterm birth, bronchopulmonary dysplasia (BPD), are coming to light through research done at Monroe Carell Jr. Children’s Hospital at Vanderbilt. The research expands understanding of the biochemical changes that can damage a premature infant’s lungs and could lead to targeted therapies for BPD.
When injured tissues attempt to repair themselves, complex developmental pathways become activated.”“The earlier infants are born, the higher their risk of BPD,” said Jennifer Sucre, M.D., assistant professor of pediatrics at Vanderbilt. “Being born early, in and of itself, creates lung injury, and many therapies used to keep babies alive, such as ventilation and oxygen treatment, have the side effect of creating injuries. When injured tissues attempt to repair themselves, complex developmental pathways become activated.”
“When injured tissues attempt to repair themselves, complex developmental pathways become activated.”
Sucre led research recently published in the American Journal of Respiratory and Critical Care Medicine revealing the role played by increased expression of a specific Wnt protein, Wnt5A, in hyperoxia injury that is thought to drive the impaired alveolarization and other clinical deficiencies of the lungs linked with BPD.
Prematurity Threatens Lung Health
Treatment with oxygen can cause a patient’s blood level of that gas to reach unhealthy levels, a condition called hyperoxia. In newborns that experience hyperoxia, the excess oxygen can damage the retina, brain and lungs. Infants that experience hyperoxia are also reported to be at higher risk of developing childhood leukemia and other cancers.
BPD can have long-term effects upon a person’s health even if the condition appears to resolve itself during infancy. In fact, premature birth is the most common cause of abnormal lung development and can lead to lifelong sequelae including asthma and COPD.
Trouble with Tissue Repair
BPD particularly affects infants born during an intermediate stage of lung development called the saccular stage, which occurs between week 23 and 32 of gestation. Wnt signaling normally peaks in a fetus at 18 weeks, and the signaling pathway is expressed at very low levels by the time of a normal birth at around 39 or 40 weeks.
In preterm babies born at the saccular stage, the researchers found that when damaged lung tissues try to repair themselves, Wnt5A becomes inappropriately activated. “If you have a strong Wnt signal when it should be silent, it disrupts development of the lungs,” Sucre said.
Sucre and colleagues previously reported aberrant Wnt signaling in the lungs of infants who died from BPD.
Three Models Used
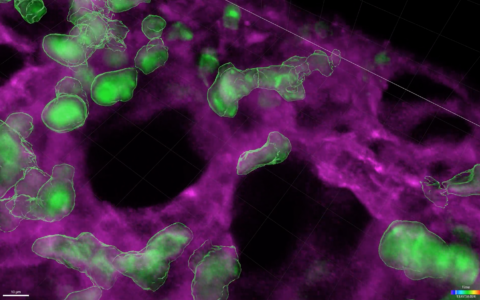
In the most recent study, Sucre’s team used three models of hyperoxia injury: 1) 3D organotypic co-cultures derived from primary human lung cells; 2) precision-cut lung slices from newborn mice, and 3) a mouse hyperoxia model.
Using 3D organotypic co-cultures, they found hyperoxia increased expression of Wnt5A, and that inhibition of Wnt5A prevented transcriptional changes associated with hyperoxia-induced BPD. Precision cut lung slices were used to show that inhibition of Wnt5a improved the ability of lung tissue to undergo alveolarization following hyperoxia exposure, a key process involved in normal lung development.
The authors also report that Wnt5A induction is influenced by NF-kb, finding that chemical inhibition of NF-kb reduced Wnt5a expression in both precision cut lung slices and mice exposed to hyperoxia.
Impact of the Research
The findings suggest that Wnt5A may be a potential target for new therapies for BPD.
“Global inhibition of the Wnt pathway would be incompatible with normal development,” the authors wrote. “However, the development of a therapeutic approach to specifically target Wnt5a represents a potential strategy to restore the normal balance of developmental signaling pathways in the lungs of preterm infants to prevent or reverse BPD.”
“The development of a therapeutic approach to specifically target Wnt5a represents a potential to restore the normal balance of developmental signaling pathways in the lungs of preterm infants to prevent or reverse BPD.”