Evidence is mounting that a common HIV drug, efavirenz, might be repurposed to treat Alzheimer’s disease.
Small amounts of the reverse transcriptase inhibitor activate the central nervous system enzyme cytochrome P450 46A1 (CYP46A1), which could help the brain metabolize mounting cholesterol during Alzheimer’s. People with Alzheimer’s disease can have altered CYP46A1 activity in their brains, which can cause cholesterol to accumulate and damage brain cells.
Pioneering research conducted at Vanderbilt University Medical Center established the role of efavirenz in CYP46A1 activation and suggests that structural modifications could enhance its potency. “Our laboratory discovered CYP46A1 could be activated pharmacologically in mice by efavirenz,” said Peter Guengerich, Ph.D., Tadashi Inagami Chair in Biochemistry at Vanderbilt. “Now, we’ve conducted mechanistic investigations to learn how to maximize this effect.”
Mechanisms of Action
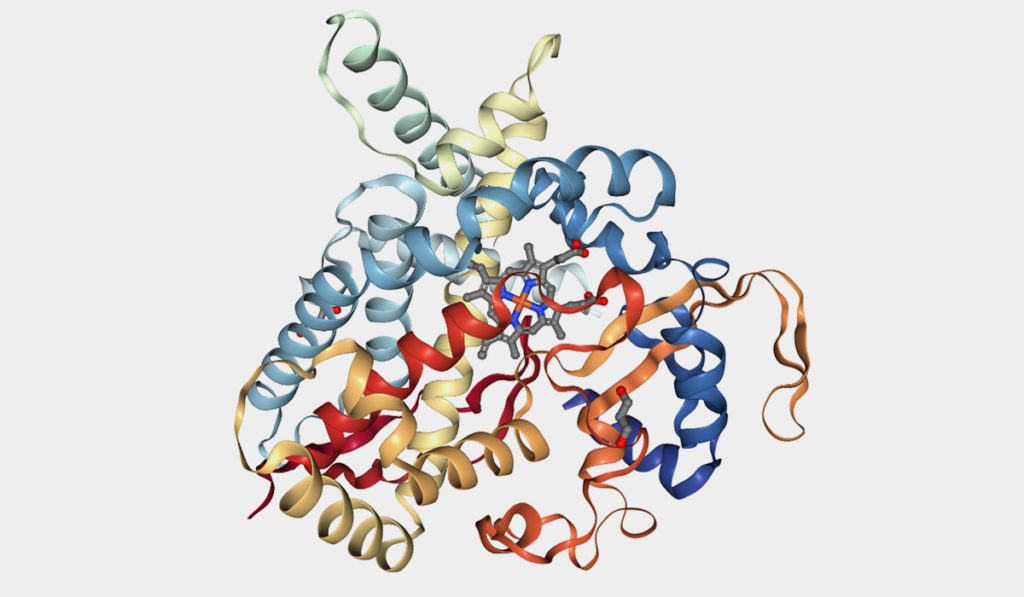
Structural studies have found a distinct binding site for efavirenz on CYP46A1. When the drug attaches to CYP46A1, it enhances the enzyme’s ability to break down cholesterol in the brain.
Researchers have also established a narrow therapeutic window whereby efavirenz activates CYP46A1. One of Guengerich’s collaborators, Irina Pikuleva, Ph.D., professor at Case Western Reserve University, found low doses of efavirenz activate CYP46A1 in mice, while high doses inhibit the enzyme. The most effective doses (0.1 mg/day/kg body weight) are nearly a hundred times lower than those prescribed to HIV patients.
Key Structures
In their most recent work, published in the Journal of Medicinal Chemistry, Guengerich and Pikuleva studied eight efavirenz-like compounds, each with a slight structural modification. “We were looking for insight into the structure-activity relationships of efavirenz for CYP46A1 activation,” Guengerich said.
One by one, the researchers mixed low doses of the compounds with recombinant CYP46A1. They added cholesterol, and measured enzyme activity and resultant metabolites. They also mapped CYP46A1 binding sites for each compound.
“The results suggest structural modifications to efavirenz to further increase CYP46A1 activation without inhibition at high compound concentrations.”
The researchers identified locations where adding a hydroxyl group increased CYP46A1 activity. They also identified uninvolved structures, which could help streamline efavirenz-like drugs in the future. Multiple isoforms proved effective, suggesting a racemic mixture of efavirenz compounds might have therapeutic benefit – unlike the specific isomers required to inhibit HIV.
“The results suggest structural modifications to efavirenz to further increase CYP46A1 activation without inhibition at high compound concentrations,” wrote the authors.
Clinical Implications
The findings are so impactful that the paper was selected as an ACS Editors’ Choice® by the American Chemical Society. It could serve as a foundation for clinical trials testing new efavirenz-like compounds as Alzheimer’s therapeutics.
“The next step includes testing the most promising compounds, first in vitro, and then, if justified, in vivo as potentially better CYP46A1 activators,” Guengerich said. “We’ve begun the process of generating these compounds for further testing.”
In the meantime, Pikuleva is involved in an ongoing clinical trial of efavirenz in people with mild cognitive impairment, which often precedes Alzheimer’s.