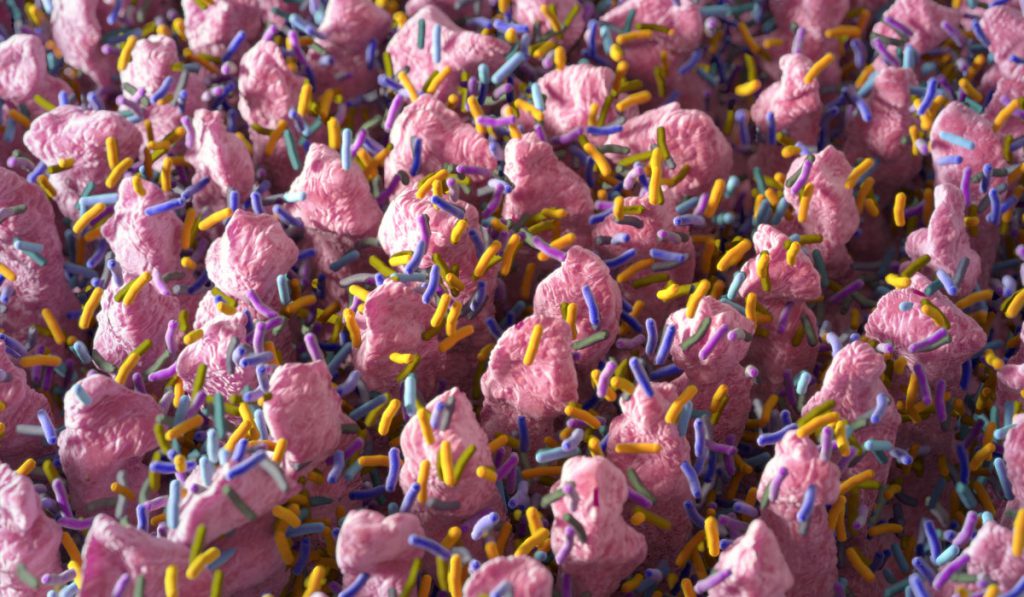
Over 90 percent of colorectal cancers (CRCs) arise from adenomas, but only a small percentage of pre-cancerous lesions will progress to cancer. A major challenge continues to be identifying those adenomas with the propensity to become cancers.
A team of Vanderbilt University Medical Center researchers is tackling this challenge, studying colorectal adenomas through the Colon Molecular Atlas Project (COLON MAP). COLON MAP is part of the Human Tumor Atlas Network, a branch of the $1.8 billion National Cancer Institute Beau Biden Moonshot initiative to tackle cancer cures. The objective is to provide a comprehensive spatial analysis of adenomas in their local environment from the pre-polyp stage forward. This includes the overlying microbiome and various underlying populations of immune cells and fibroblasts.
“There are more bacteria, more organisms, in the colon than there are cells throughout our bodies… Those organisms have a big impact on the health of our colon.”
“It’s a really exciting project because we know that there are more bacteria, more organisms, in the colon than there are cells throughout our bodies,” said Timothy Geiger, M.D., chief of the Division of General Surgery at Vanderbilt. “Those organisms have a big impact on the health of our colon, but we have a poor understanding of what that impact is, how it works.”
Spearheading the Project
The initiative is led by co-primary investigators Robert Coffey, M.D., a cell biologist who directs the Vanderbilt Epithelial Biology Center (EBC) and leads the Vanderbilt-Ingram Cancer Center Specialized Program of Research Excellence (SPORE) in Gastrointestinal Cancer, Ken Lau, Ph.D., a systems biologist at Vanderbilt with special expertise in single cell RNA sequencing, and Martha Shrubsole, Ph.D., a cancer epidemiologist specializing in precancerous lesions.
Shrubsole is leading efforts to collect and analyze biospecimens provided by 1,200 patients between 40 and 75 years old with healthy and diseased colons. “We have the opportunity, for the first time, to deeply phenotype the polyps and local environment at the single-cell level, and also integrate these molecular signatures with other lifestyle and etiologic biomarkers to better understand mechanisms and strategies for colorectal cancer prevention,” she says.
A New Approach
To date, research has focused on the genetic mutations that lead to CRC proliferation, and carcinogenic factors that contribute to them. Little focus has been on the progenitor cells themselves, what makes them vulnerable to mutation and what process they undergo before becoming invasive.
“This is a systematic effort to longitudinally collect and perform genomic profiling of premalignant lesions as they progress towards frank malignancy or spontaneously regress,” Coffey said. “Comprehensive characterization of premalignant lesions is needed to formulate risk-stratification and intervention strategies.”
The investigators are studying multiple phenomena emerging as CRC contributors. “For example,” Coffey said, “Epithelial cells make a layer of mucous in which tumor-promoting bacteria may lodge and invade—into and through the epithelial cells—to contribute to the development and progression of the adenoma.”
“One of the biggest challenges is how to build computational models that account for all the spatial, temporal, functional, cellular and molecular variables.”
The goal is to understand which lesions are most likely to progress to cancer, which kinds of precancerous lesions and what sites are most fruitful to study, and how premalignant lesions are best measured. “One of the biggest challenges is how to build computational models that account for all the spatial, temporal, functional, cellular and molecular variables that contribute to carcinogenesis,” Shrubsole says.
Creating A Comprehensive Atlas
The project will generate a Pre-Cancer Atlas (PCA), formally described as “a collection of molecular, cellular, subcellular, structural, and functional maps that provide integrated information of the tumor and its immediate microenvironment in relation to tumor initiation, expansion of premalignant lesions, progression to invasive cancer and metastasis,” per the authors of The Making of a PreCancer Atlas: Promises, Challenges, and Opportunities.
Its mission is to use retrospective biospecimens from existing cohorts to inform promising technologies. These technologies can then be applied to prospectively collected longitudinal biospecimens, building an atlas that elucidates all the relevant characteristics of precancer pathways. “By identifying new markers of early detection, this atlas should generate multiple intervention targets for preventing the development of cancer,” Shrubsole said.