The robust success of off-label ketamine rocked the medical field when it became recognized as a lifesaver for patients with severe symptoms such as suicide ideation. Ketamine’s success also sparked intense research into how it works, with the goal of developing drugs that mimic its trigger of glutamate release while avoiding its psychotic side effects. Such a development could move indications for this class of drugs from “treatment-resistant” only to first-line therapy.
In new findings published in Neuron, Jeffrey Conn, Ph.D., director of the Vanderbilt Center for Neuroscience Drug Discovery, and post-doctoral fellow Max Joffe, Ph.D., recently developed and tested two glutamate receptor antagonists. They demonstrated the power of the antagonists to facilitate glutamate transmission to the prefrontal cortex. In animal models, these antagonists mimic the anti-depressant-like effect that ketamine has in the brain.
Targeting Brain Circuitry
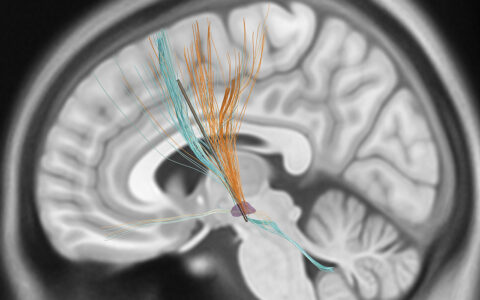
Ketamine is thought to produce its anti-depressant effect by increasing activity of glutamate neurons, triggering higher glutamate release in a specific brain circuit – the thalamocortical loop. A malfunctioning/maladaptive thalamocortical loop is complicit in a wide range of movement, cognition and mood disorders. “This research is another scientific step toward a drug that will selectively enhance thalamocortical transmission without over-exciting the circuit in the way ketamine does,” Conn said.
“This research is another scientific step toward a drug that will selectively enhance thalamocortical transmission without over-exciting the circuit in the way ketamine does.”
Optimizing Glutamate
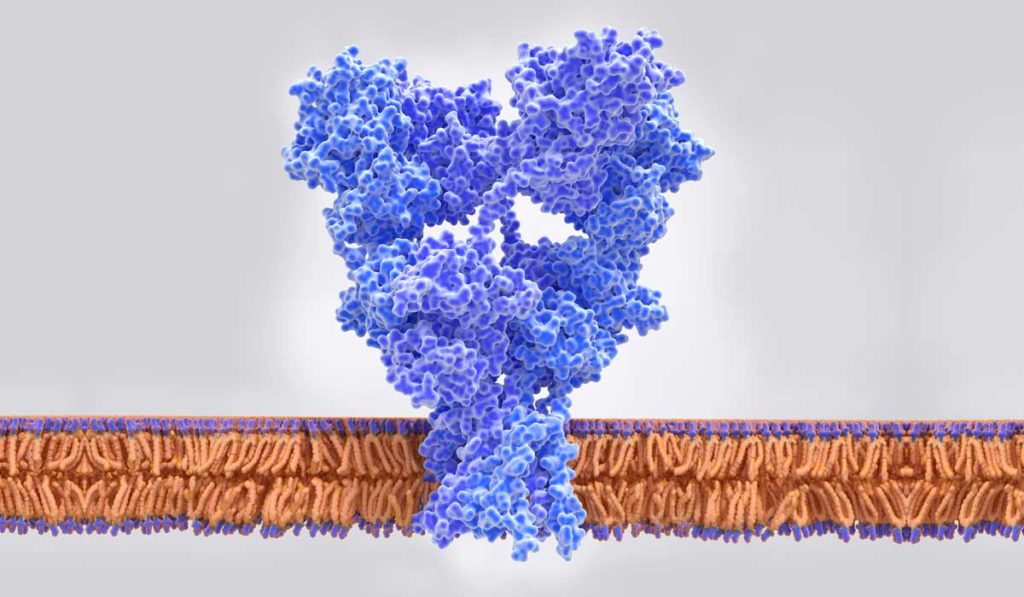
Conn is a leading researcher in “metabotropic” glutamate receptors in the brain. Conn and Joffe focused their study on mGlu2 and mGlu3 receptors, which have an inhibitory effect on glutamate through fundamentally different mechanisms. They hypothesized that the selective inhibition of mGlu2 or mGlu3 would potentiate prefrontal cortex excitatory transmission, thereby producing an anti-depressant effect.
Using preclinical models, they developed separate compounds that serve as antagonists for mGlu2 and mGlu3 to block glutamate reuptake. Previous research had not differentiated between the two. “We found that both receptors impact transmission at the synapse between the thalamus and the prefrontal cortex, one pre- and one post-synapse,” Conn said. Both antagonists induced anti-depressant-like effects through related, but divergent, mechanisms.
Adding Clinical Options
While these findings suggest biomarkers for drug development, Conn says the work is still early, basic research. “The compounds we developed to impact mGlu2 and mGlu3 did not exist before now, but they are not optimized as drug candidates for humans.” He foresees a multimillion-dollar effort to move such compounds into clinical development. The earliest a drug could reach the market would be in 11 or 12 years, he says.
Conn, Joffe and their team are also involved in more advanced trials for drugs that impact Parkinson’s dystonia, schizophrenia and Alzheimer’s. “The role of receptors in various regions of the brain is a mainstay of our research,” Conn said.
“The neurochemistry of glutamate receptors is one of the more critical aspects in determining healthy or unhealthy brain function.”
The new study serves as a foundation, Conn said. “What we know now is that the neurochemistry of glutamate receptors is one of the more critical aspects in determining healthy or unhealthy brain function/regulation. That is a springboard for much of our work to come.”