Use of checkpoint inhibitor immunotherapies by eligible cancer patients has grown from less than two percent in 2011 to 43 percent in 2018. The best available clinical measure of effectiveness of immunotherapy drugs today is PET evidence of tumor regression or growth. However, current PET scan techniques produce insights later than is ideal and can miss critical changes, including metastases.
In a new collaboration between GE Healthcare, Vanderbilt University Medical Center, and Indi Molecular, researchers will aim to develop PET radiotracers that provide an earlier, full-body read on a patient’s immune response to therapy. This would enable quicker assessment of immunotherapy’s impact by showing the quantity and sites of T cell recruitment to the tumor microenvironment.
“We are very excited about this ambitious project. We would like to move a target compound through the pipeline within two years – from basic chemistry, to screening and imaging, and into first-in-human studies,” said Charles Manning, Ph.D., Ingram Professor of Cancer Research at the Vanderbilt-Ingram Cancer Center, who is leading the project.
Filling a Void in Immunotherapy Assessment
Currently, there are no imaging biomarkers that can predict a patient’s response to immunotherapy. Clinicians can only view tumor growth or regression by looking at the quantity of cell growth promoters, like glucose or glutamine, that is taken up by the tumor. An option to perform earlier and more specific assessments could inform decisions to switch therapies or stay the course.
“The field of immunotherapy is moving very rapidly, and largely without sufficient predictive and quantitative biomarkers.”
Developing such a biomarker for immunotherapy would require tracking T cells. Manning and his team will start by targeting a specific receptor, CD8. A small molecule will be labeled with the radioisotope F-18 and delivered to bind with CD8 sites, illuminating T cells throughout the body.
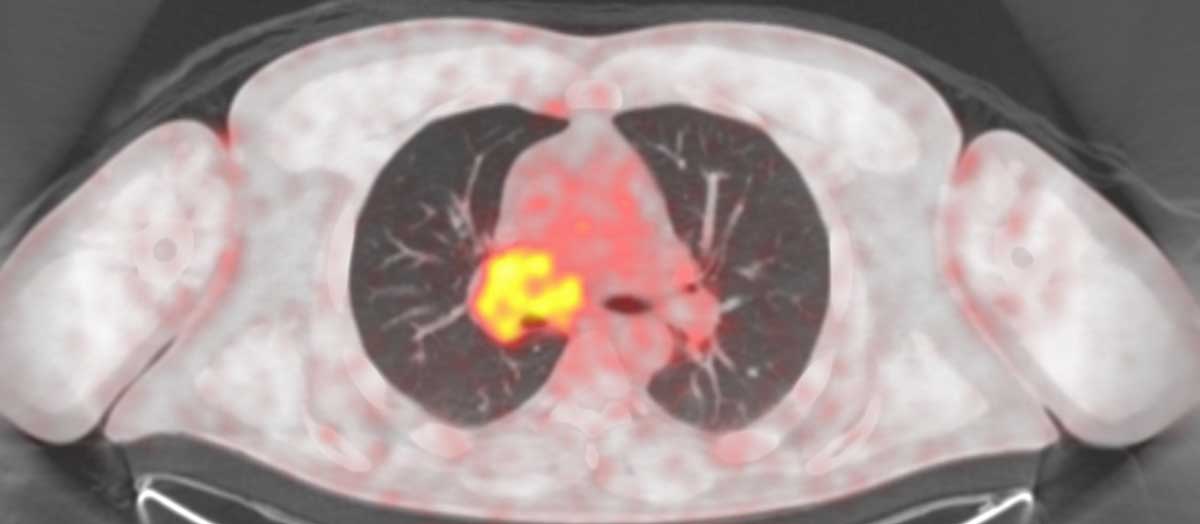
The new PET scans would also quantify the body’s T cell reserves in organs like the spleen to predict sufficient or insufficient immune response. Accumulation of T cells in other organs, a particular concern in avoiding cardiomyopathy, could be assessed. Multiple tumors could be imaged synchronously. Small areas of metastases would illuminate.
“Between Indi Molecular’s platform and Vanderbilt’s resources, we are uniquely positioned to do this work,” Manning said. “Indi Molecular has the screening technology to identify peptides that target unique features of proteins.”
Manning says the biggest challenge will be to prioritize which peptide is most effective, instead of using a group of peptides to hit the target. “The endpoint of this research is going to be about prioritizing something to bridge to a first-in-human study.”
Pieces of the Puzzle
The imaging tracer development will draw heavily on the expertise of Jeffrey Rathmell, Ph.D., director of the Vanderbilt Center for Immunobiology and an authority on T cell behavior in the tumor microenvironment. Immune system testing will be performed by Ann Richmond, Ph.D., Ingram Professor of Cancer Research at Vanderbilt, using mice with human immune systems.
“We have all the pieces of the puzzle needed to develop, test and select the right tracers in a short time frame,” Manning said.
Manning’s ultimate vision is to develop “tool boxes” of distinct, translatable PET tracers for immuno-oncology that can be used clinically and in researching and developing new therapies, he said.