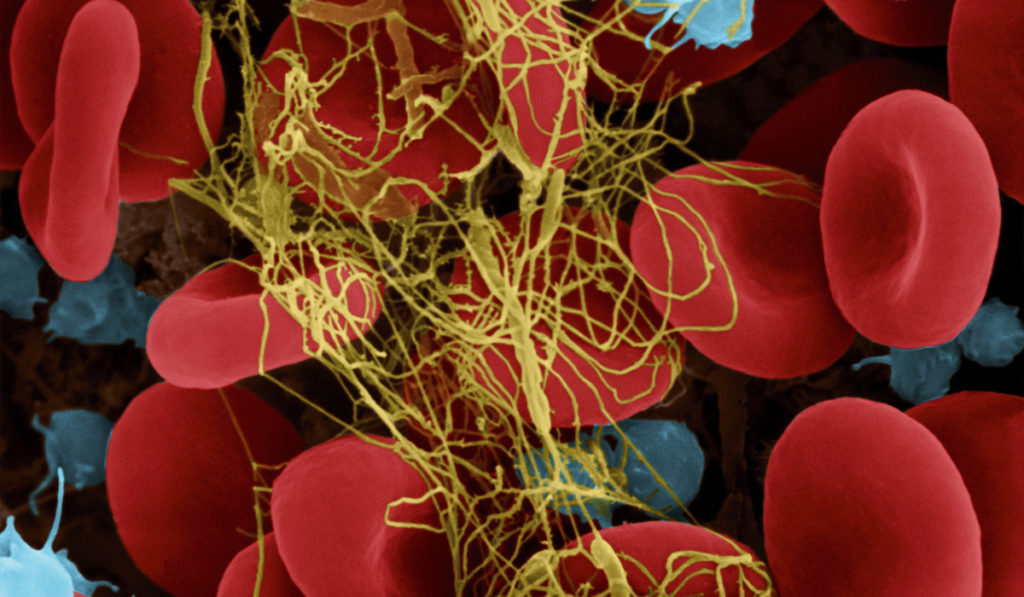
Researchers at Vanderbilt University Medical Center are working to harness plasmin, a powerful reparative protease present in blood, to promote tissue repair.
In a series of previous studies, Jonathan Schoenecker, M.D., Jeffrey Mast Chair of Orthopaedic Hip and Trauma Surgery at Vanderbilt, and colleagues have shown that controlling the timing and extent of plasmin activation may be key to preventing blood loss during surgery, while also promoting appropriate repair of injured tissues, such as bone and muscle.
“Robust plasmin activity is essential during repair, but equally important is to limit plasmin activity in the early timepoints of injury to assure containment of the injured tissue,” said Schoenecker.
“Robust plasmin activity is essential during repair, but equally important is to limit plasmin activity in the early timepoints of injury to assure containment of the injured tissue.”
Given plasmin’s variable roles, Schoenecker and his team have established a directed animal model to study the specific steps of the pathologic continuum reported in JCI and the Journal of Bone and Mineral Research and funded by the National Institutes of Health and Department of Defense.
Plasmin Activation Upon Injury
Injury is followed by a survival phase and a reparative phase. During the survival phase, hemostasis and inflammation contain the damage and prevent infection by producing a fibrin web. The reparative phase involves a reparative inflammatory response that methodically removes fibrin and damaged tissue while also stimulating tissue repair.
To initiate angiogenesis and repair, inflammatory and progenitor cells activate plasmin, the primary protease of the fibrinolytic system. A healthy plasmin response snips away at the fibrin web, allowing cells to navigate through the damaged tissue.
In a severe injury, however, plasmin is inappropriately activated during the survival phase. This not only removes the essential fibrin web at the wrong time, causing significant blood loss, but is also associated with multi-organ failure, poor tissue repair and degenerative pathologies during the repair phase.
A Role for Plasmin in Tissue Homeostasis
Plasmin plays another significant role in maintaining tissue homeostasis. For example, in less acute injuries such as those occurring in bone from daily activity, plasmin promotes removal of fibrin. In this context, inefficient plasmin activity leaves behind fibrin, which although great for stopping bleeding, can cause chronic inflammation and degeneration of local tissue.
“Fibrin becomes a ‘matrix splinter’ instigating inflammatory cells to perpetually try to remove it, causing extreme collateral damage to the surrounding tissue.”
“Essentially, fibrin becomes a ‘matrix splinter’ instigating inflammatory cells to perpetually try to remove it, causing extreme collateral damage to the surrounding tissue, such as bone loss leading to osteoporosis,” Schoenecker said (as reviewed in Blood).
“We were somewhat myopic when we started looking at this because we were studying a single disease process and focused timepoints,” added Schoenecker. “However, once we had pinpointed the plasmin paradox in orthopedic trauma, we and others outside of orthopaedics started to put our stories together and we realized that precise management of fibrin and plasmin within the acute phase response is essential for virtually all tissues.”
One case was a paper published on Alzheimer’s disease that mirrored the one the Schoenecker team had published on osteoporosis. In that study, investigators discovered that inefficient plasmin activity in the Alzheimer’s brain leads to fibrin accumulation which promotes neurovascular damage and amyloid deposits.
Controlling Plasmin Clinically
As part of the continuum investigation, Schoenecker and his team are looking at patient profiles to confirm their findings and to understand when plasmin activation is advantageous and when it is dangerous. From these profiles, they hope to use activators and inhibitors to control plasmin activity, limiting it to where it is needed to maintain tissue homeostasis and repair, and preventing it in situations that cause bleeding, cancer and infections.
The impact of controlling plasmin clinically is best exemplified by its now ubiquitous use in orthopaedic surgery, Schoenecker said. “The use of plasmin inhibitors during the survival phase of orthopaedic surgery has dropped the need of blood transfusions to the point that certain surgical procedures which once required days in the hospital, can now be done on an outpatient basis, because of the reduced risk of bleeding.”
The team anticipates that judicious activation of plasmin in repair may have a similarly beneficial effect in promoting tissue repair.