A slew of research around the role of microtubules in insulin release from beta cells has yielded mixed results, said Kathryn Trogden, Ph.D., postdoctoral fellow and cell biologist in the laboratory of Irina Kaverina, Ph.D., professor at Vanderbilt University.
“Microtubules have been found to have positive, negative and no effect on insulin secretion. Their role has been pretty controversial. Not many people have looked at this in the past couple of decades.”
In a study published in Current Biology, Trogden and her collaborators helped put the controversy to rest. Said Trogden, “It’s more complicated than we originally thought.”
A Microtubule Cage
Microtubules comprise cell scaffolding. As they polymerize and grow inside beta cells, microtubules can form a cage around a cell’s contents. As they depolymerize, cellular contents are released. The denser the microtubules, the more tightly a cell holds onto its contents.
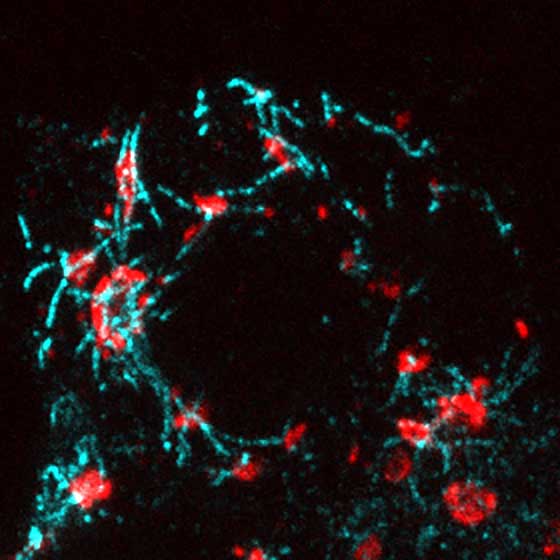
“In beta cells, most microtubules come from the Golgi, and they’re much denser and more multi-directional than in other cell types. That allows them to hold insulin granules inside,” Trogden explained. “When you stimulate beta cells with glucose, immediately you start losing microtubules, and insulin granules are released.”
A Dual Role
“Microtubules are being regulated by the same pathway that regulates insulin secretion.”
The new study found not only does glucose cause microtubules to depolymerize in beta cells, but it also triggers the growth (“nucleation,” Trogden explained) of new microtubules. Specifically, glucose triggers microtubule nucleation from the Golgi, not centrosomes (where microtubules involved in cell division originate). The group previously identified the Golgi as a microtubule site of origin, but the connection to insulin secretion wasn’t yet clear.
“We wanted to know, why is the cell making new microtubules when it’s trying to lose them? As it turns out, microtubules are being regulated by the same pathway that regulates insulin secretion,” Trogden said.
Using live cell imaging and other techniques, the researchers uncovered a long-term role for microtubules in beta cells, in addition to their short-term “cage” role. They showed Golgi-derived microtubules are required for the biogenesis of new insulin granules. Glucose (via cAMP/EPAC2 signaling) stimulates the growth of microtubules from the Golgi. As the microtubules begin to jut out from the Golgi, they carry with them budding insulin granules. At the same time, microtubules are depolymerizing at the other end to release fully-formed granules.
“Microtubule nucleation is absolutely having a positive effect on insulin secretion,” Trogden concluded.
A Clue for Diabetes Treatment
Trogden notes that during diabetes, microtubules are much denser inside beta cells. This makes it more difficult for beta cells to release insulin.
“Regulation of microtubule nucleation at the Golgi could be affecting this density,” Trogden said. “It will require more research, but there seems to be some sort of issue with microtubule density in these cells. They are trying to secrete insulin granules and are just very inefficient.”
One possibility could be that beta cells are “over-nucleating” microtubules at the Golgi, contributing to the dense network. Too much nucleation could affect beta cells’ ability to effectively balance insulin storage and secretion.
Said Trogden, “If you don’t have a proper amount of microtubules in these cells, you’re going to have an issue with insulin secretion in some way.” Trogden and other researchers in the Kaverina laboratory are now looking into ways to help stabilize microtubule density in beta cells during type 2 diabetes.