Immune checkpoint therapy has led to long-term remission for some patients with advanced cancer. However, many cancer patients experience side effects and either do not respond or have only short-term responses to checkpoint therapy, which targets inhibitory receptors on T cells.
A new study published in Nature offers clues as to why blocking inhibitory receptors on tumor-specific T cells (TST) may not always work. Mary Philip, M.D., assistant professor of Medicine in the Division of Hematology and Oncology at Vanderbilt University Medical Center, and co-senior author Andrea Schietinger, Ph.D., of Sloan Kettering Institute, found the thymocyte selection-associated high-mobility group box protein, called TOX, is expressed at high levels in dysfunctional TST in mice and humans.
“We hope to make T cells resistant to cancer-induced dysfunction.”
“We are trying to understand what is happening in those first few days when the T cells first encounter abnormal cells that have cancer-causing mutations. With this knowledge, we hope to make T cells resistant to cancer-induced dysfunction and improve immunotherapy for cancer patients,” Philip said.
Building on Previous Findings
The TOX study follows an investigation published in Nature by Philip and colleagues in 2017, which showed that CD8 T cells specific for an antigen expressed in liver cancer cells differentiate to an epigenetically-encoded dysfunctional state. The differentiated cells exhibit hallmarks of TST dysfunction including the expression of inhibitory receptors and loss of effector cytokine production. As a next step, Philips wanted to investigate regulatory mechanisms behind such hallmarks of TST dysfunction.
New Results
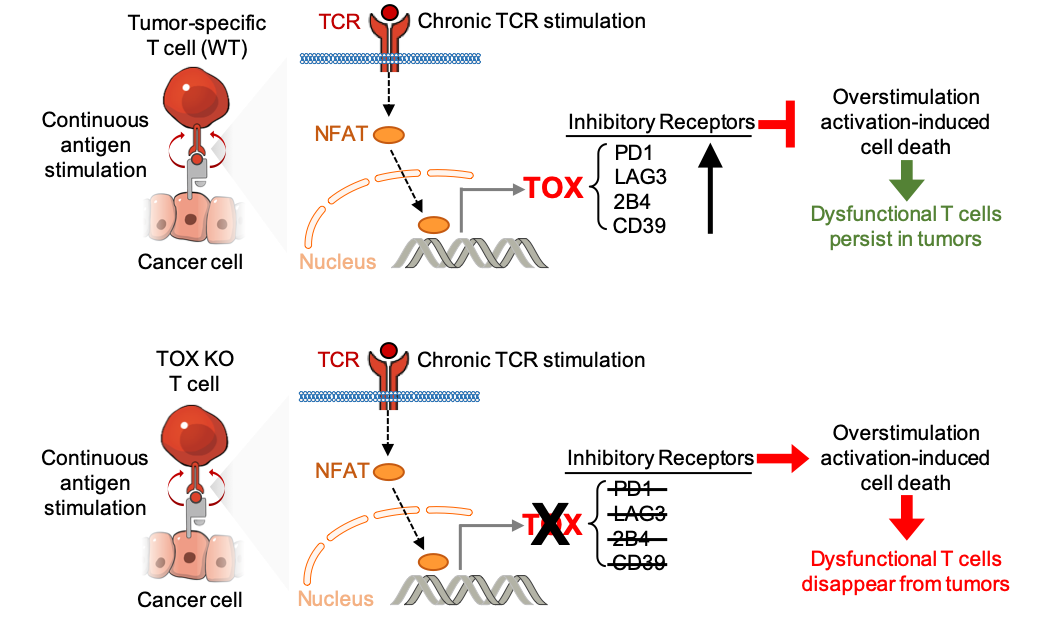
In the new study, the investigators looked at various models of chronic infection and tumors and found that TOX controls the high expression of inhibitory receptors, such as PD1, LAG3, 2B4 and CD39 on dysfunctional TST. The team then deleted TOX from TST to see if that would restore normal function.
Despite their normal, “non-exhausted” immunophenotype, TOX-deleted TST remained dysfunctional, revealing that the regulation of inhibitory receptor expression is uncoupled from the loss of effector function. While TOX-deleted CD8 T cells differentiated normally in response to acute infection, TOX-deleted TST failed to persist in tumors. The team hypothesizes that the TOX-induced exhaustion program serves to prevent T cell overstimulation and activation-induced cell death in chronic antigen stimulation.
“Taking off the brakes is not enough to restore the killing capacity of anti-tumor cells. In fact, T cells need the brakes to avoid getting over-activated and dying,” Philip said.
Developing a New Research Model
Philip’s research team has begun development of an organoid model of the liver. The goal is to induce cancer in liver organoids, then add T cells and other immune cells to understand how the immune system responds to developing tumors. This in vitro model will allow three-dimensional observation of immune-cancer interactions at the single-cell level at the earliest stages of cancer development, which is difficult to carry out in mouse models.
While other organoids have been developed, including stomach and liver models, “what hasn’t been done yet is trying to get liver cancer to form in vitro,” Philip said. “Can we recapitulate in a dish the carcinogenesis that we see in our mouse models? We are looking at T cell interactions within these organoids.”