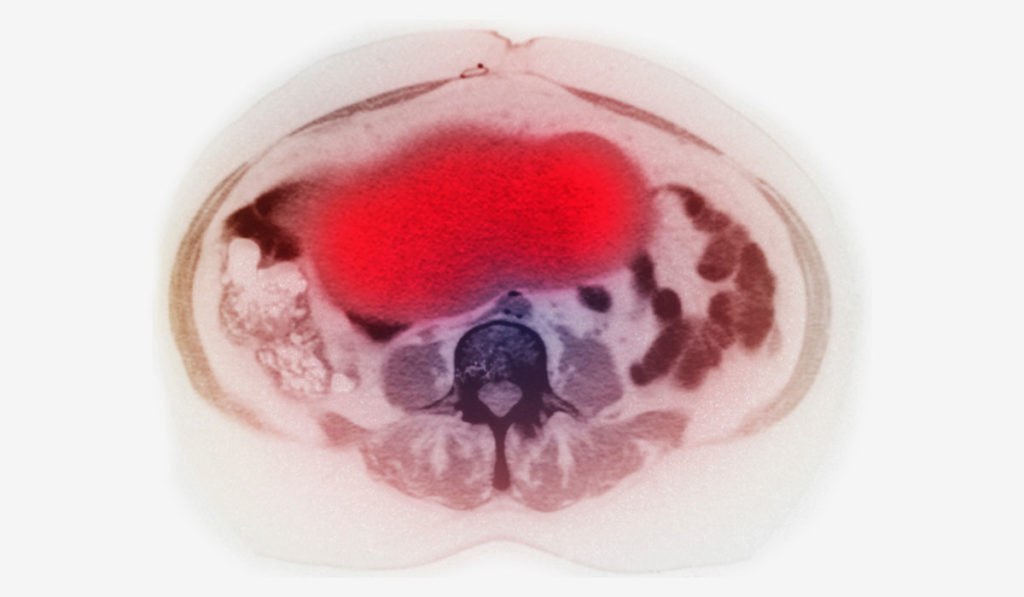
Early detection represents a critical strategy against ovarian cancer, the fifth most common cause of cancer-related death in women in the United States. While most ovarian cancer is diagnosed at an advanced stage at which the 5-year survival rate is less than 30 percent, early detection increases the 5-year survival rate to about 93 percent.
“Ovarian cancer is well-treated if detected early. But it usually isn’t,” said Lawrence Marnett, Ph.D., dean of basic sciences and professor of biochemistry, chemistry and pharmacology at Vanderbilt University School of Medicine. “Serous ovarian cancer is the most malignant and most common form of ovarian cancer and it spreads through the abdomen early so that it isn’t detected until at an advanced stage.”
In a recent study reported in ACS Omega, Marnett and colleagues, including Md. Jashim Uddin, Ph.D., research associate professor in the Department of Biochemistry at Vanderbilt, designed a cyclooxygenase (COX)-1 targeted imaging agent for early detection of ovarian cancer.
“We were hoping to use molecules that are expressed in ovarian cancer as targets for imaging those tumors as they develop,” Marnett said.
Cyclooxygenases: Markers of Early Lesions
Cyclooxygenase plays a role in the generation of bioactive lipids, some of which contribute to the growth and dissemination of tumors. There are two forms of the enzyme, COX-1 and COX-2. COX-2 is well-recognized as a marker of inflammation and carcinogenesis.
“When there’s an early premalignant stage that one can detect, typically COX-2 is expressed in those lesions,” Marnett explained. “This was first discovered in the colon and has been recapitulated in many different kinds of cancers.”
Marnett has had a longstanding focus on imaging COX-2. “We’ve developed a number of PET imaging agents to detect COX-2, which has provided us a very strong base for understanding the chemistry and structural biology of inhibitors interacting with that target.”
“For reasons we don’t really understand, COX-1 is the form of the enzyme that is expressed in ovarian cancer.”
“For reasons we don’t really understand,” Marnett added, “COX-1 is the form of the enzyme that is expressed in ovarian cancer.” Previous research by Marnett and others revealed that COX-1 is expressed at high levels in type II ovarian tumors and is associated with reduced overall survival.
A Novel Chemistry Scheme
The new radiopharmaceutical developed by the team, referred to as [18F]FDF, is a COX-1 inhibitor labeled with fluorine-18, a radioisotope necessary for PET imaging. “It was a major accomplishment and used a lot of chemistry to do was looks pretty simple on a piece of paper,” Marnett said.
To start, an established COX-2 inhibitor, Vioxx, was redesigned to selectively bind COX-1 instead of COX-2.
“We knew from a lot of work that had been done in the literature, and work in our own laboratory, that if you take the methylsulfone substituent off Vioxx and replace it with other functional groups, it actually selectively binds to COX-1,” Marnett said.
Incorporation of the fluorine radioisotope was trickier.
“This is tough chemistry,” Uddin said. “The precursor is not stable under the conditions that you have to use to introduce fluorine-18.” Uddin attached a substituent to stabilize the chemical scaffold and developed a novel Lewis-acid catalyzed chemistry scheme to incorporate fluorine-18.
Validating the Compound
Uptake of [18F]FDF was fivefold higher in tumors than in normal muscle tissue, indicating that signal intensity from [18F]FDF can distinguish a COX-1 expressing tumor from the background noise of COX-1 expression in the peritoneal cavity. The compound also exhibits other key requirements for use as a PET imaging agent: minimal defluorination, minimal off-target organ uptake, and a plasma half-life of 1.2 hours.
Two preclinical mouse models of ovarian cancer were used to evaluate [18F]FDF in the recent study. Maximal uptake of [18F]FDF occurred one hour post-injection in a subcutaneous xenograft model, and in less than five minutes in a more physiologically relevant peritoneal tumor model.
“The point here,” Uddin explained, “is that when it is physiologically relevant, and you have vascular connections with the tumor, then you can deliver material pretty easily and quickly to the tumor.”
Future studies are in the pipeline to validate [18F]FDF for diagnostic targeting of ovarian cancer, including metabolism and toxicology studies.