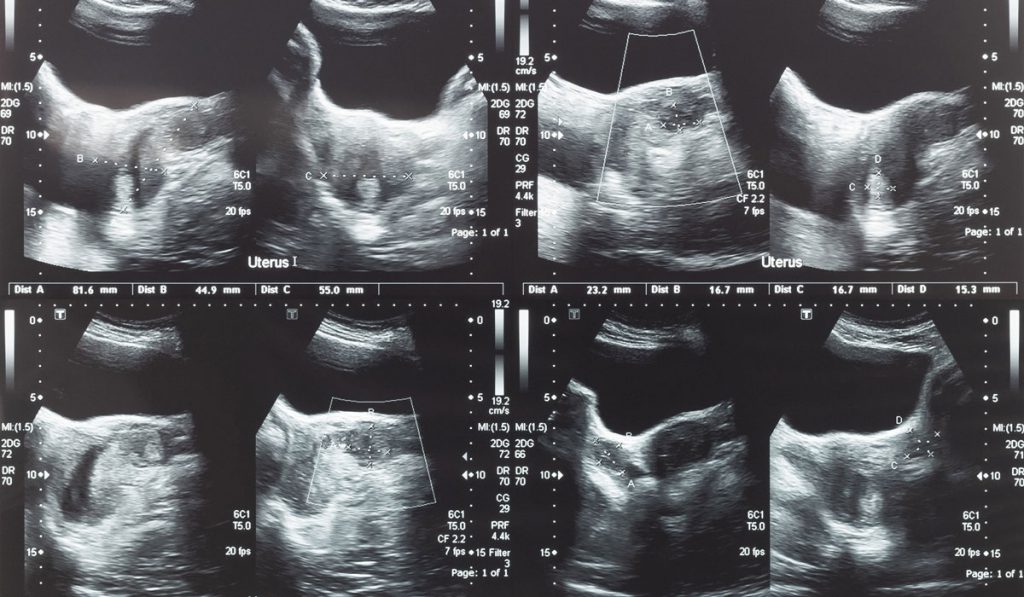
Uterine fibroids account for about one-third of hysterectomies in the U.S. and are found in at least 25 percent of hysterectomy specimens. Pre-1990, vaginal hysterectomy, laparoscopic-assisted vaginal hysterectomy, or abdominal hysterectomy/myomectomy were the primary surgical interventions. By the early 1990s, laparoscopic techniques were well-established and laparoscopic hysterectomy and myomectomy became available to patients with large fibroids. Benefits included lower estimated blood loss, reduced pain, reduced incidence of wound complication, shorter convalescence and better cosmetic appearance.
“Fibroids don’t necessarily mean you need a hysterectomy,” said Ted Anderson, M.D., vice chair for Clinical Affairs and Quality in the Department of Obstetrics and Gynecology at Vanderbilt University Medical Center. “Today, 75% of patients desire minimally invasive treatment and 50% desire uterus-preserving treatment. With laparoscopic myomectomy, we are able to offer that desirable alternative; the challenge has been to find the best option for tissue extraction.”
The Morcellator Controversy
The first electromechanical morcellator—employing a sharp spinning hollow tube that cuts the fibroid tissue through small port incisions and excises it through the tube—was introduced in 1991 and has been an effective aid to laparoscopic surgeries. However, morcellation poses some risk: mechanical risk of collateral tissue damage and risk of dissemination of cancerous tissue, including endometrial cancer, endometrial stromal cell sarcoma/STUMP and leiomyosarcoma (LMS).
In 2014, the FDA ruled that power morcellation was “contraindicated” in the majority of women. Most hospitals issued a ban on uncontained morcellation. In 2016, the first FDA-approved product specifically for contained morcellation (within a specimen bag) was introduced, but many hospitals are still not using any type of morcellation in uterine surgery.
In 2016, Anderson and 45 colleagues from top U.S. and international hospitals issued an open letter to the FDA in the Journal of Minimally Invasive Gynecology regarding morcellation use. The group challenged data used to calculate uterine LMS incidence and the prognosis for women with morcellated LMS.
“The problem has always been the inability to detect cancerous fibroids preoperatively and to predict which patients may have predisposition to cancer.”
“We can argue about the true incidence and age-related risk of encountering a leiomyosarcoma, but the real issue is that risk does exist,” he emphasized. “The problem has always been the inability to detect cancerous fibroids preoperatively and to predict which patients may have predisposition to cancer.”
Next in Leiomyosarcoma Research
“As we approach the five-year mark of the morcellation controversy, there is still a lot we don’t know,” Anderson said. Outstanding questions include:
- What is the real incidence of LMS among women undergoing surgery for presumed fibroids?
- Does the risk associated with tissue dissemination vary by route of morcellation?
- Do specimen containment and retrieval bags eliminate or reduce risk?
- What is the clinical outcome with LMS after laparoscopy and morcellation compared with intact tissue removal by laparotomy?
- Does intact removal of uterine fibroids really offer any benefit over morcellation during myomectomy or is there still increased risk when encountering an undiagnosed LMS?
Anderson hopes that ongoing research will lead to a better understanding of pre-operative diagnosis and reducing risk for surgical management for uterine fibroids that may contain LMS.
“We are pursuing parallel paths,” Anderson said. “We’re finding better ways to predict and identify these cancerous fibroids so we can know when to avoid morcellation and otherwise modify our surgical technique to minimize risk, and we’re working to develop mechanisms that overcome the possibility of leaving tissue behind when some form of morcellation is necessary.”