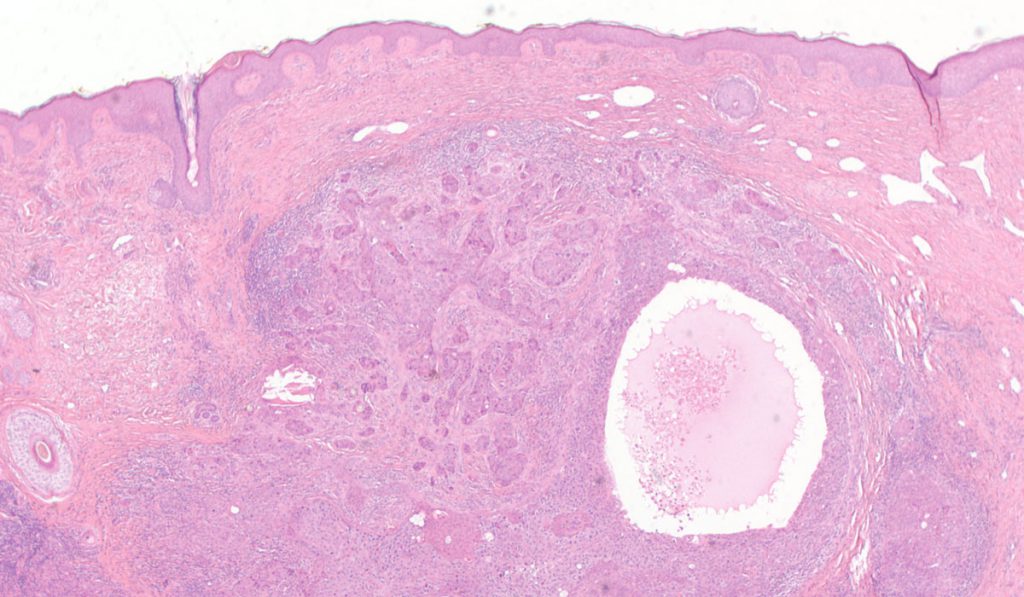
For the first time, researchers have mapped the genomic landscape of a very rare tumor of the hair shaft, called a malignant proliferating tricholemmal tumor (MPTT).
These rare neoplasms begin in the external hair sheath, are often confused with squamous cell carcinoma (SCC) and have the potential to metastasize. Tailored treatment for metastatic MPTT is not standardized, as only 27 cases have been reported in the literature, said Christine Lovly, M.D., a medical oncologist and physician scientist at Vanderbilt-Ingram Cancer Center.
Lovly recently provided details about a 28th case in a report published in NPJ Precision Oncology. The case report is the first to include metastatic MPTT genotyping, which will help inform future, more precise treatment regimens for this rare tumor.
An Unexpected Case
The report describes a 58-year-old, previously healthy woman who presented to her primary care provider to have a cyst on her scalp removed. The cyst had been present for over a decade and pathology suggested SCC, with potential for PTT. She underwent plastic surgery to have the growth removed, but it recurred locally and in the posterior cervical lymph node, followed by multiple mediastinal lymph nodes. She underwent two surgeries to remove the growths, and received another SCC diagnosis along the way, informing her treatment regimen.
Eventually, pathology revealed MPTT and the woman benefitted from a phase 1 trial of apelisib, but developed pneumonia and opted to end aggressive treatment. After the patient’s death, Lovly collaborated with a team of researchers—including medical oncologists, surgeons, pathologists, radiologists, bioinformaticians and laboratory scientists—to learn from autopsy results.
Clues from Genotyping
“By testing the tumor for possible mutations, we were actually able to find a target that we knew a lot about in other cancers.”
Initial DNA sequencing of the woman’s tumor had revealed a PI3K mutation, which allowed her to enroll in the apelisib trial (a PI3K-alpha-selective inhibitor).
Said Lovly, “In this case, there was no standard of care therapy for this patient, but by testing the tumor for possible mutations, we were actually able to find a target that we knew a lot about in other cancers.”
To gain a deeper understanding of molecular alterations that may drive MPTT, the researchers also performed whole genome sequencing. They compared genetic data from the woman’s tumors to her healthy liver tissue. Ten of her tumors harbored the PIK3CA H1047R mutation, plus numerous other somatic, exonic, copy number, and structural variants.
Across 18,120 single nucleotide polymorphisms, insertions and deletions, the researchers identified 38 that had never before been reported in the Catalogue of Somatic Mutations in Cancer (COSMIC). They also found 238 copy number variations in the tumors. Genes altered in the woman’s metastatic MPTT did not significantly overlap with genes associated with the external hair sheath or a cutaneous SCC—suggesting a specific genetic “signature” for metastatic MPTT.
Support for Precision Medicine
“While rare, we have learned critical biological and clinical information from the thorough sequencing and analysis of this metastatic MPTT,” said Vanderbilt trainee and one of four first authors listed on the study’s first author and Vanderbilt trainee, Jean-Nicolas Gallant, Ph.D.
The new data suggest SCC and MPTT are genetically distinct, which could help future patients avoid misdiagnoses and receive precise, appropriate care. Genetic signatures identified by the researchers could serve as a reference for other oncologists to support MPTT diagnoses. Simple PIK3CA genetic testing could be one way to differentiate MPTT from SCC, which is less likely to have PIK3CA mutations.
Other patients with metastatic MPTT are likely to benefit from genotype-driven therapies made possible by the new data. “We hope by writing this paper, it helps the next patient who has this rare disease,” Lovly said. “Now, we can bring precision medicine to the treatment of these rare tumors. And we deeply appreciate the outstanding teamwork and collaboration¹ that we shared to bring this study to fruition.”