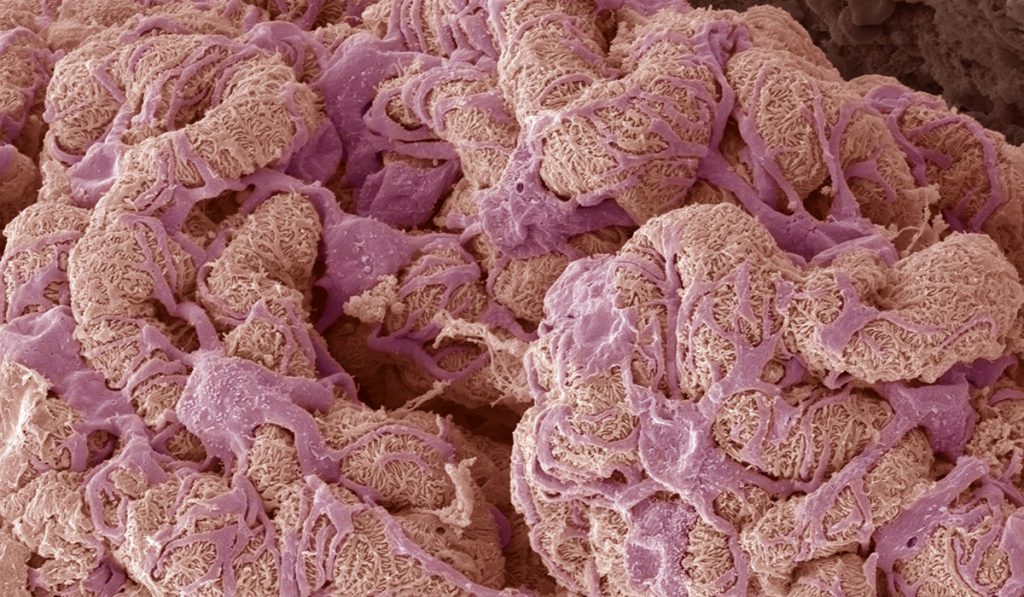
New research could help explain mechanisms that underlie rare conditions including Goodpasture’s disease and Alport syndrome. Both conditions can damage glomerulus basement membranes, impairing how kidneys filter metabolic waste.
The basement membrane may be one of the earliest structures to evolve in multicellular organisms, according to Aaron Fidler, Ph.D., nephrology researcher at Vanderbilt University Medical Center. Fidler is studying proteins that attach to basement membranes and surrounding extracellullar matrix to help date their origins.
In a study selected as an “Editor’s Pick” in the Journal of Biological Chemistry, Fidler helped trace the origin of one particular matrix protein—Goodpasture antigen-binding protein (GPBP)—and found it is one of the oldest. Understanding this ancient protein could help explain how basement membranes evolved, and how they function in the body today, Fidler says. “The basement membrane and extracellular matrix that makes up the foundation of epithelial tissues is more complex than we ever thought.”
An Evolutionary Discrepancy
Single-celled organisms turned multicellular more than 600 million years ago. Cells began to form networks and gain specialized functions, including basement membranes—organized cell networks that still underlie all epithelium in the body.
Over time, the gene encoding GPBP began to be transcribed into several isoforms. Humans have three: GPBP-1 is found outside cells, GPBP-2 is found inside and GPBP-3 is membrane-bound. GPBP diversity has spurred controversy over how cells began to organize into multicellular structures.
The researchers looked for different GPBPs in ancient organisms to find the oldest isoform. Fidler and a team led by Vanderbilt professor Billy Hudson, Ph.D., compared transcriptomic and genomic data to piece together the GPBP evolutionary timeline. They found GPBP-2 in unicellular choanoflagellates and filastereans. GPBP-1 and GPBP-3 were found in multicellular chordates and marsupials, respectively, that evolved later.
The findings, wrote the authors, place GPBP-2 “at the dawn of multicellularity”—one of the first components of basement membranes present in the body today.
A Deeper Understanding of Epithelium
“These findings add to the growing understanding of the basement membrane and how the proteins that make up the space outside of cells function to build multicellular tissues,” Fidler said.
In addition to ancestry, the researchers also looked at cellular distributions of GPBP-2. They found GPBP-2 both inside and outside of ancient unicellular organisms called cnidarians, with kinase activity akin to that of human GPBP-2. The similarities suggest that even the earliest lifeforms had tremendous genetic complexity, and multifunctional proteins.
“These findings add to the growing understanding of the basement membrane and how the proteins that make up the space outside of cells function to build multicellular tissues”
Understanding the evolutionary origins of GPBP helps explain how it behaves in human diseases involving the basement membrane, such as Goodpasture’s disease. “It’s like if you had to fix a car,” Fidler explained. “It’s a lot easier to start by studying a Ford Model T than a modern Lamborghini. Lessons on basic car mechanics are translatable to even the most sophisticated models.”
Broadening Scope to Other Proteins
Going forward, the researchers plan to study how GPBP may have driven evolution of other key basement membrane components, especially collagen IV. Collagen IV provides the scaffold upon which GPBP and other proteins attach in human basement membranes.
Previous work by Hudson’s team has shown during Goodpasture’s disease collagen IV is defective, and this causes the autoimmunity that ultimately attacks the basement membrane. GPBP may be a key player in how this disease develops.
“The interactions of proteins in the basement membrane and the chances for dysfunction are massive,” Fidler said. “Exploring these components and understanding how they interact—and what they do individually—are vital steps toward future therapies and treatments.”