Stereotactic body radiation therapy (SBRT) is a noninvasive procedure in which a radiation oncologist directs a highly focused external radiation beam to destroy a targeted tissue area with great precision. Already used to treat a variety of tumors and certain movement disorders, SBRT is only now being investigated to treat cardiac problems.
Because the heart is a moving target, it presents special challenges. Interdisciplinary teams are responding to this challenge by leveraging SBRT in conjunction with innovative motion compensation techniques—with early trials suggesting strong benefits.1
At Vanderbilt University Medical Center, radiation oncologist Eric Shinohara, M.D., and electrophysiologist William Stevenson, M.D., are launching a trial investigating SBRT for patients with ventricular tachycardia (VT) that failed catheter ablation. “We’ve seen the promising results with SBRT in several early studies led elsewhere,” Shinohara said. “We’re excited to test this using our own unique approach.”
Filling a Persistent Need
“With catheter ablations… use is limited if the VT substrate is in deep intramural locations or when catheter access is precluded by a mechanical valve, or possibly intracavitary thrombus.”
Patients with VT commonly have a structural heart disease that creates scarring and in turn disrupts electrical conductivity. Catheter ablation has been the primary intervention if medication fails, yet many patients who have an ablation continue to suffer frequent VT episodes. Patients with refractory VT often undergo multiple ablations, are heavily medicated, and may have an implantable cardioverter-defibrillator (ICD) that can increase mortality risk and hasten heart failure.
SBRT promises a potentially strong alternative. Its non-invasive nature means those with the most recalcitrant hearts may be treated effectively.
“One of the challenges with catheter ablations is that their use is limited if the VT substrate is in deep intramural locations or when catheter access is precluded by a mechanical valve, or possibly intracavitary thrombus,” Stevenson said. “Additionally, an invasive ablation approach can present challenges for patients with comorbidities or an ICD already in place.”
Adapting SBRT for Cardiac Applications
When SBRT is delivered to a cancer patient, the target may be stationary or its movement can be limited by controlling respiration. Typically, the radiation oncologist can use CT, MRI or PET images to identify the tumor.
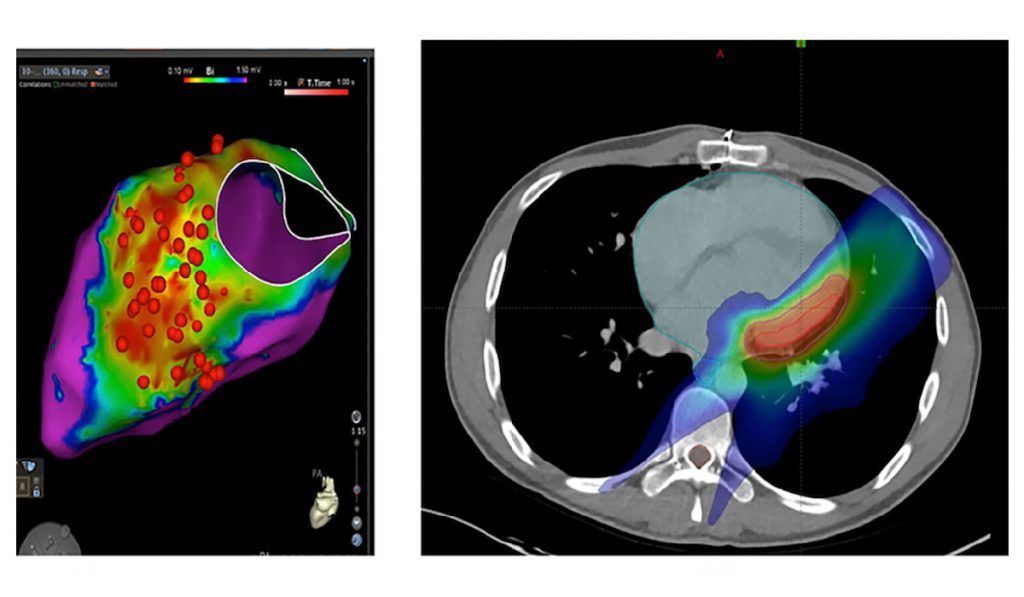
To tackle the challenge of targeting abnormal tissue in a beating heart, electrophysiologists and cardiologists must go beyond anatomical imaging. In one earlier study of SBRT for VT, researchers created maps of cardiac electrical activity and overlaid them onto a thoracic, non-contrast, gated CT image to select the area targeted for radioablation. Using this technique, 18 of 19 patients who had been refractory to invasive ablation experienced significant improvement in symptoms. Median VT episodes fell from 119 in the six months prior to the procedure, to three in the six months after the procedure.1
“At Vanderbilt we have substantial expertise in localizing areas of electrically abnormal tissue causing VT,” Stevenson said. “Part of our research efforts will focus on methods to improve localization and targeting of the arrhythmogenic areas.” The team integrates information from cardiac mapping studies and electrocardiograms of the arrhythmia with a 3D facsimile of the heart created with ultrasound imaging, to define the regions causing VT. This 3D representation is then overlaid onto a CT image to guide SBRT.
“We absolutely rely on the help of the cardiology team because they not only provide the echo image, they are actually making an electrical map of the heart to show which areas are dysfunctional,” Shinohara said. “We depend on them to clearly define the target areas when we create our treatment plans.”
Advancing the Technique
“This is a new technique and we need to learn much more about it.”
At Vanderbilt, SBRT will be initially offered to patients whose arrhythmia has not been controlled with conventional ablation. However, as the technique evolves, Stevenson hopes it may replace primary ablation in many cases. “With this technique the patient’s arrhythmia can be ablated without inserting any catheters into the heart,” he said. “This is a new technique and we need to learn much more about it.” The damage produced by radiation can potentially extend to structures outside the heart and may emerge many years later. Ongoing research will help optimize methods for targeting the arrhythmia areas and achieving effective and safe ablation.
The Vanderbilt study is currently under Institutional Review Board approval. Shinohara and Stevenson hope to enroll patients as soon as this spring.