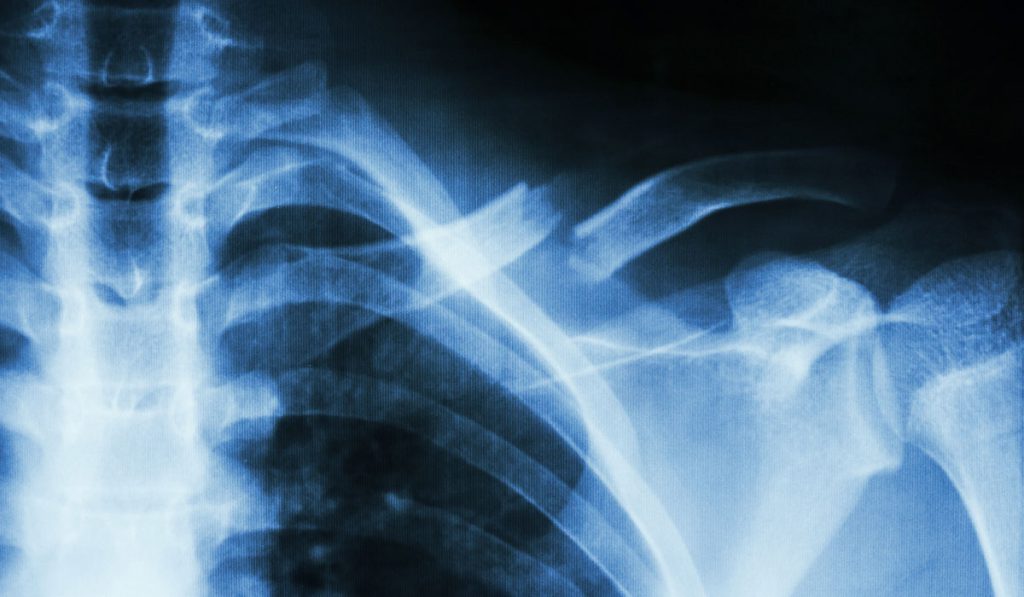
Patients with type 2 diabetes may be twice as likely to suffer a fragility fracture as their age-matched, non-diabetic counterparts. While the systemic impact of diabetes on bone fracture risk is broadly acknowledged, mechanisms for how the bone becomes compromised are not well-understood.
“We still need to develop a better understanding of the differences that put type 2 diabetes patients at higher fracture risk, as well as how diabetes disease progression impacts bone integrity,” said Jeffry Nyman, Ph.D., a researcher with Vanderbilt’s Center for Bone Biology. Nyman is leading a large, multifaceted study on bone fracture risk with broad implications for patients with type 2 diabetes.
For older patients in particular, the stakes of increased bone fracture risk are considerable. Fractures in older adults are associated with significant morbidity and mortality. Independent of age, those with diabetes take longer for their fracture to heal.
A Detailed Analysis of Bone Properties
Nyman and his team will examine bone matrix in live subjects and cadaveric samples to probe why patients with type 2 diabetes are at higher fracture risk. Cadaveric bones will undergo destructive measurements of strength and various mechanical properties that characterize fracture resistance. Live participants will undergo non-invasive or minimally invasive tests.
Specific research aims of the study are to:
- Test the predictive potential of different technologies for assessing bone strength in age-matched cadavers from donors with and without type 2 diabetes;
- Study the biomechanical reasons for high fracture risk in all subjects; and
- Identify molecular changes in the bone matrix that impact fracture resistance.
Nyman’s team will leverage four existing technologies in innovative ways:
- DXA dual X-ray absorptiometry (DXA): DXA is the gold standard for assessing osteoporosis. The study will benchmark new technologies to DXA-bone mineral density for their utility in detecting a potential increase in fracture risk with type 2 diabetes.
- RAMAN spectroscopy: A technique developed for clinical applications at the Vanderbilt Biophotonics Center and not previously applied for bone matrix quality assessments in subjects, RAMAN spectroscopy will be used to direct light to the surface of the bone to generate a graphic representation of molecular vibration.
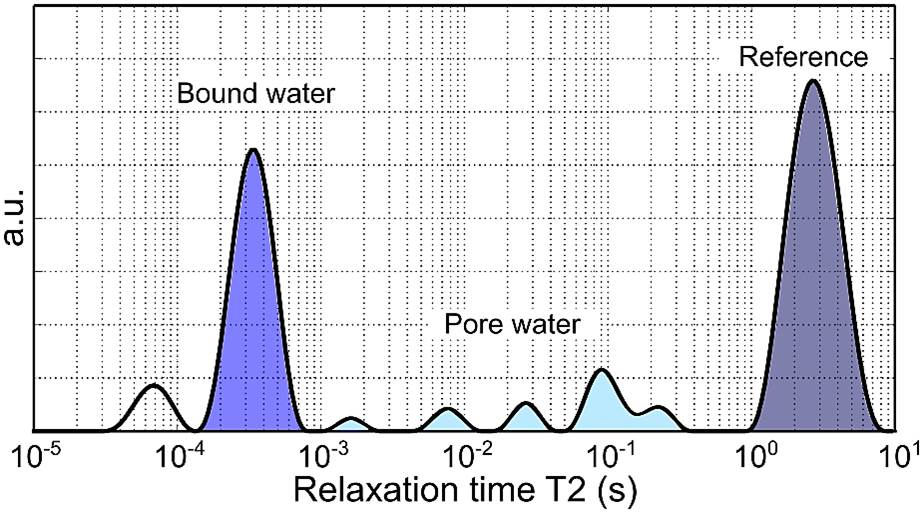
- Proton nuclear magnetic resonance (NMR) relaxometry: A highly sensitive means of assessing water content in bone, NMR underpins MRI. MRI methods were developed at the Vanderbilt University Institute of Imaging Science to generate images of collagen-bound water and pore water (in microvascular channels) in subjects. The study will determine whether this new bone measurement may differ between patients with and without type 2 diabetes.
- Osteoprobe: A minimally invasive technique that measures bone material strength index by creating a microindentation in the periosteum. It has proven useful in identifying osteoporosis for post-menopausal women. The study will examine its utility with men and women with type 2 diabetes.
“With this combination of live subjects and bone samples, and this application of a variety of technologies, we believe we can generate rich data sets that will shape a broad understanding of diabetic bone disease,” Nyman said.
Toward an Evidence-Based Model of Diabetic Bone Fracture Risk
Previous explanations of increased bone fracture risk with type 2 diabetes have not focused on specific changes in bone composition. One hypothesis has been that type 2 diabetes patients fall more because of retinopathy and resultant poorer vision. “But that doesn’t fully explain their higher incidence of fracture,” Nyman said.
Nyman says diabetic nephropathy could also be a player in disease-related changes in bone structure. “With bone having the greatest calcium reservoir, the kidney has a lot to say about what bone is doing, so diabetic nephropathy is bound to have a huge impact on fracture risk,” he said.
However, Nyman currently favors another explanation: sugar-mediated modification to bone matrix, combined with other complications like microvascular disease. “You can put bone ex vivo in sugar and watch the changes taking place,” Nyman said. “Even people with well-controlled diabetes have glucose spikes. With frequency, these can prevent adequate nutrients from reaching the bone, increase its porosity and modify the organic matrix.”
As the research leads to more evidence-based understanding, Nyman looks forward to seeing the development of targeted care plans for fall avoidance and other interventions for type 2 diabetes patients at higher fracture risk. “If we better understand the changes in the bone that affect fracture resistance, there is a clearer path to therapies that help block or slow those changes,” Nyman said.