Cell culture is evolving to include multi-dimensional systems that recapitulate human tissues more precisely than flat Petri dishes. Organoid technology has allowed researchers to grow 3D mini-brains and hearts. Now, organ-on-a-chip systems are revealing new insights about human reproduction.
“Organ-on-a-chip takes multiple cell types, grows them in the right 3D arrangement with low overhead fluid volume so they can chemically communicate, to better mimic the in vivo environment of a particular developing organ system,” said Shane Hutson, Ph.D., chair of Physics and Astronomy at Vanderbilt University.
Hutson is director of the Vanderbilt-Pittsburgh Resource for Organotypic Culture Models for Predictive Toxicology (VPROMPT). VPROMPT has five main projects addressing different aspects of reproductive health. One of the projects, “validating a fetal membrane on a chip model for characterizing reproductive toxicant exposure risks,” is led by Vanderbilt investigator Kevin Osteen, Ph.D., along with co-investigators Kaylon Bruner-Tran, Ph.D., and David Aronoff, M.D., also of VUMC.
Building a Fetal Membrane Model
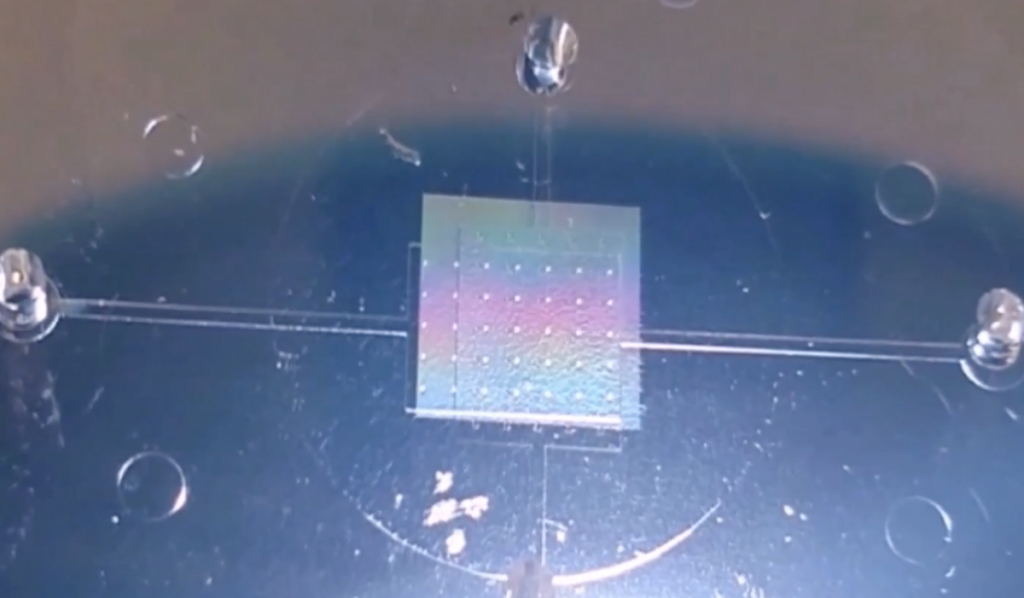
“We were charged with, from scratch, building a model. Along with researchers from MIT, we created chips with microfluidic cell chambers and pump systems that continuously perfuse cells growing inside.”
With funding from the Environmental Protection Agency, the researchers collaborated to develop an organ-on-a-chip model that mimics the female reproductive tract. The initial study focused on how manmade toxicants cross the fetal membrane and cause adverse pregnancy outcomes.
“We were charged with, from scratch, building a model,” said Osteen. “Along with researchers from MIT, we created chips with microfluidic cell chambers and pump systems that continuously perfuse cells growing inside. The fluid that flows through them is similar to the volume that would flow through an organ in the human body.”
The chips contain stackable chambers to allow researchers to “co-culture” multiple cell types in one environment. Researchers can seed the chambers with primary cultures of human fetal membranes that grow into a 3D model, demonstrating cellular interactions that occur in vivo. The chips can also be adapted to model the menstrual cycle, the maternal-fetal interface, and effects of disease on these processes.
First Applications
In their first proof-of-concept study, the researchers used the model to simulate temporal hormone changes that occur during a 28-day menstrual cycle. They successfully grew stromal cells that differentiated into functional decidual cells akin to those found in the endometrium during pregnancy.
Now, Osteen and colleagues are using the chips to test the effects of reproductive toxicants – and ways to combat them. Said Osteen, “We’re able to put placental cells and maternal cells on the chip, use the same bioactive agents we use in nutrition, like omega 3 or resveratrol therapies, and do a proof of principle of when you would intervene in a human to reduce the risk of preterm delivery.”
The researchers are also running immune cells through the chips to see how they interact with cells and hormones found in the reproductive tract. “Bringing the immune system and the endocrine systems together is really the big value of the chip,” said Osteen. “You can study things that are traditionally studied in different laboratories by different groups.”
Bridging the Gap Between Animal Models and Humans
Organ-on-a-chip technology could significantly reduce the need for animal models. Instead of exposing mice to toxicants, researchers can expose dynamic cell culture systems. The chips may also more accurately model human immunobiology.
“The physiology of mice and woman are similar in some ways but, there are important differences. For example, mice don’t menstruate, so they don’t spontaneously develop endometriosis,” explained Osteen. Endometriosis is a common reproductive disorder of women, and frequently leads to infertility.
With chip models of menstruation in hand, the researchers can study how toxicants, infections and other environmental factors contribute to adverse reproductive outcomes. Said Osteen, “We can also stimulate cells to secrete more of what they should, or less of what they shouldn’t, in terms of what we know is associated with a successful pregnancy.”
Next Steps
Osteen and Bruner-Tran also have Veterans Administration funding to support another organ-on-a-chip model, investigating toxicants that soldiers are exposed to during war. “We suspect there’s a familial epigenetic risk that’s playing out and we’re seeing that with reduced male fertility in soldiers,” said Osteen.
Osteen is also collaborating with researchers at Vanderbilt-Ingram Cancer Center to model mouse and human omentum on chips. Ovarian, pancreatic, endometrial and colon cancer cells all migrate to the omentum. Osteen is hopeful the chips will reveal what drives this movement, and a new way to model cancer cell interactions with the omentum.