Degenerative diseases of the central nervous system involve early degradation of axon function that often precedes outright degeneration. Pruning of dendrites and their synapses represents a potential driver of axonopathy by depriving neurons of necessary activity. Identifying new therapeutic targets for neurodegenerative disease requires understanding how neurons respond to stress and whether this response includes adaptive mechanisms to slow progression.
In glaucoma, the world’s leading cause of irreversible blindness, optic nerve degeneration involves early stress to retinal ganglion cell (RGC) axons from sensitivity to intraocular pressure (IOP). This sensitivity also influences survival of RGC dendrites and their excitatory synapses in the retina.
Determining the Mechanisms of RGC Damage
In a newly published study, David Calkins, Ph.D., vice chair and director for Research at Vanderbilt Eye Institute, and his laboratory team found that elevations in ocular pressure caused an increase in ganglion cell excitability, including response to light, even in cells with substantial dendritic pruning. This adaptation arose from voltage-dependent mechanisms in the axon and may help maintain signaling to the brain to preserve vision.
Dendritic pruning occurred early, by two weeks of elevation, and independent of whether the RGC responded best to light onset or offset. Pruning, axon dysfunction and deficits in visual acuity did not progress further between two and four weeks of elevation. These results suggest that neurodegeneration in glaucoma involves an early axogenic response that counters IOP-related stress to slow progression and maintain signaling to the brain. Thus, short-term exposure to elevated IOP may pre-condition the neural system to further damage.
“We found that the mechanism that causes this response actually preserves vision for a brief period of time, despite the onslaught of stress in glaucoma,” said Calkins. “We think we can develop new therapies based on this mechanism to keep the optic nerve signaling, which will maintain vision even as a disease progresses.”
Calkins was recently awarded a National Eye Institute RO1 grant as part of the “Audacious Goals” initiative to research this hypothesis further. This is one of several NEI grants Vanderbilt Eye Institute has received in support of its Regenerative Visual Neuroscience initiative to study glaucoma and other degenerative eye diseases.
Interleukin-6: A Promising Therapeutic Target
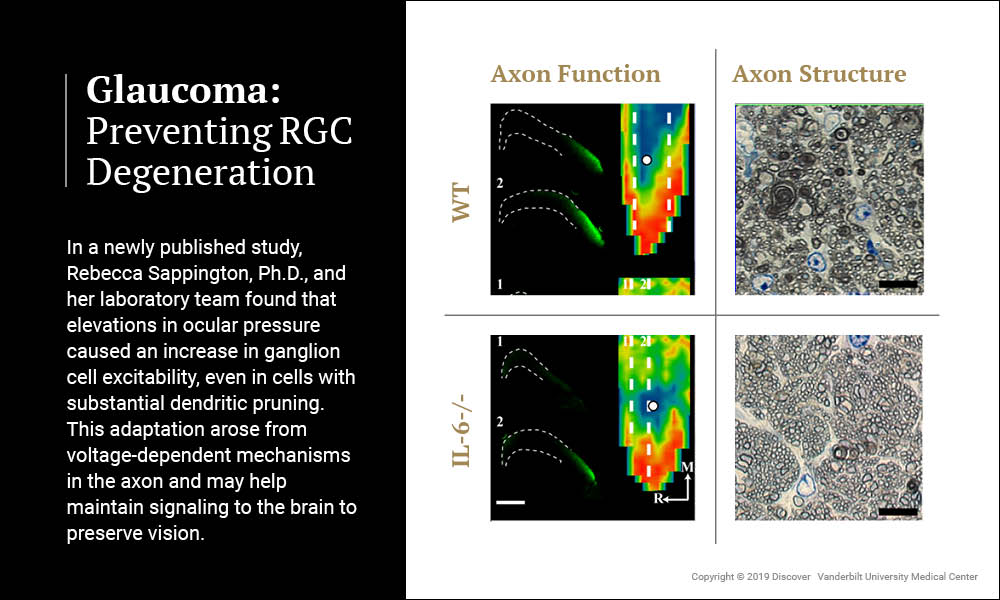
Rebecca Sappington, Ph.D., associate professor of Ophthalmology and Visual Sciences at Vanderbilt, has received a 5-year renewal of a National Eye Institute RO1 grant to study the cytokine interleukin-6 (IL-6), a promising therapeutic target for preventing retinal ganglion cell (RCG) degeneration in glaucoma. Her recent study, published in Frontiers in Neuroscience, indicates that IL-6 plays a major role in optic nerve degeneration and blindness.
“In efforts to develop therapeutics for glaucoma, as in neurodegeneration itself, timing is everything.”
Sappington and her laboratory team artificially induced glaucoma in wildtype mice and mice lacking the gene for IL-6. Mice lacking IL-6 exhibited the same degree of molecular transport dysfunction in axons of the optic nerve as wildtype mice. However, unlike wildtype mice, this functional impairment did not progress to structural degeneration of the optic nerve and vision was subsequently preserved.
These findings indicate that different mechanisms control the functional and structural components of axonopathy in glaucoma. Furthermore, they identify IL-6 signaling as a component of the mechanism that specifically leads to structural degeneration of axons. The study confirms the notion that the time between functional impairment and structural degeneration in axonopathy is a viable therapeutic window for glaucoma and identifies IL-6 as a target for such therapeutics.
“Neurodegeneration is not an event. It is a process… a series of events,” said Sappington. “In efforts to develop therapeutics for glaucoma, as in neurodegeneration itself, timing is everything.”