Improving diagnosis and treatment of small cell lung cancer (SCLC) hinges on better understanding the disease’s progression. One significant challenge to date has been lack of adequate tumor biopsy specimens.
“SCLC is a highly lethal malignancy for which there’s been no therapy change for more than three decades. It’s critical that we find better ways to diagnose and treat patients with this disease,” said Christine Lovly, M.D., Ph.D., assistant professor in the Division of Hematology and Oncology at Vanderbilt University Medical Center. Lovly and a research team at Vanderbilt are investigating liquid biopsies as an alternative approach to monitoring progression of SCLC.
Testing Liquid Biopsies
Tumor biopsy specimens are limited and often don’t provide enough tissue to complete comprehensive genomic profiling of SCLC tumors. In addition, repeat biopsies throughout the course of the disease is not the standard of care for patients with SCLC outside of a clinical trial.
“The goal of our study [published in the January 2018 issue of the Journal of Thoracic Oncology] was to find ways to monitor disease progression, in a longitudinal fashion, in patients with SCLC using liquid biopsies,” said Lovly. “We hypothesized that blood-based testing would be able to circumvent some of the significant limitations that the field faces with tumor sample acquisition.”
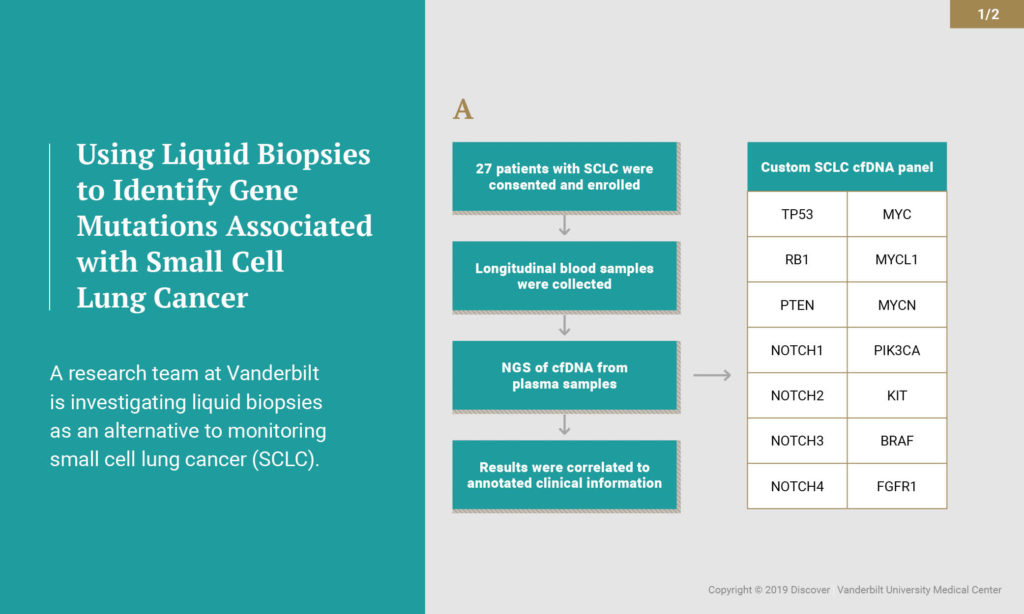
Working with researchers at Resolution Bioscience (Bellevue, Washington), Lovly’s group used circulating tumor DNA (ctDNA) to monitor the progression of the disease using liquid biopsy blood-based assay. Patients with SCLC who prospectively consented, regardless of stage or treatment, had additional blood samples taken at every standard of care blood test – before, during and after treatment. Over 26 months, the investigators studied 140 blood samples from 27 patients.
“This study shows us that we can detect ctDNA in the plasma of patients with SCLC at very high levels.”
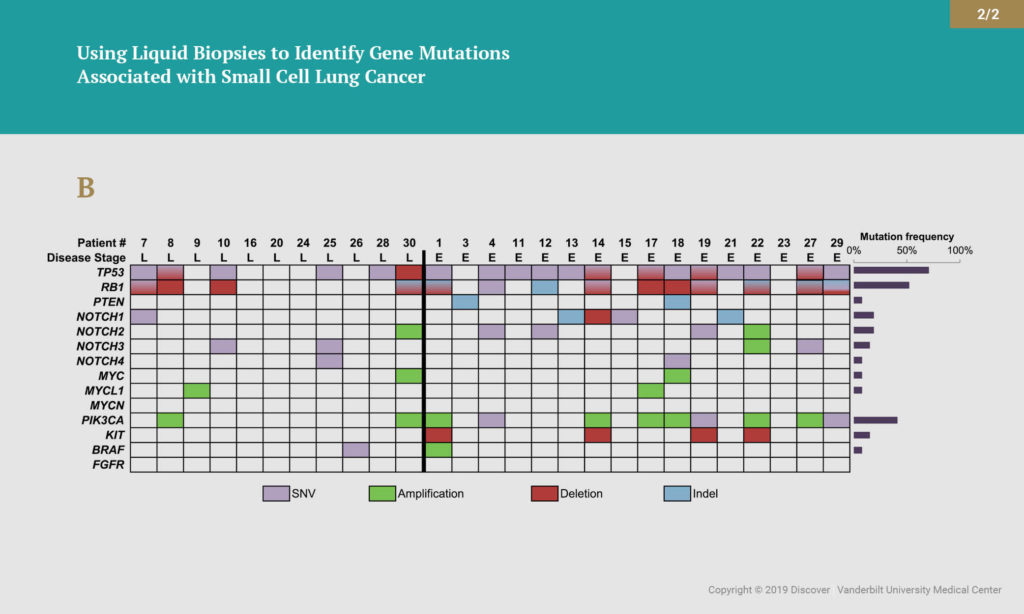
The researchers isolated ctDNA from the patient plasma and sequenced the ctDNA for 14 genes that are frequently mutated in SCLC. The 14-gene assay can detect single nucleotide variants, copy number alterations and insertions/deletions. Overall, disease-associated mutations were detected in 85 percent of these samples. More than half (59 percent) of those patients had advanced disease. The most common mutations occurred in the TP53 and RB1 genes. The group also found genomic alterations in PTEN, NOTCH1-4, PIK3CA, KIT and BRAF. In several cases, analysis of ctDNA provided evidence of disease relapse before it could be detected by standard radiographic imaging.
“This study shows us that we can detect ctDNA in the plasma of patients with SCLC at very high levels,” Lovly said. “This tells us blood-based testing is feasible in patients with SCLC. We also learned that amount (allele frequency) of tumor associated mutations detected decrease or increase as the patient responds or fails to respond to therapy, respectively. And finally, the use of ctDNA monitoring has the potential to add valuable insight in cases with ambiguous CT scan findings.”
Next Steps
The data now need to be put into prospective analyses so that doctors can learn more about how liquid biopsies may best be utilized to identify gene mutations associated with SCLC, with the end goal of improving patient care.
“This serves as a foundation for us to say that ctDNA testing should be used in clinical trials that are ongoing in patients with SCLC,” Lovly said.
The study was led by first authors Karinna Almodovar, Ph.D., and Wade Iams, M.D., with Lovly as principal investigator. Other contributing investigators included Catherine Meador; Zhiguo Zhao; Sally York, M.D.; Leora Horn, M.D.; Yingjun Yan; Heidi Chen, Ph.D.; Yu Shyr, Ph.D.; Jennifer Hernandez; Lee Lim and Christopher Raymond (Resolution Bioscience).